INTRODUCTION
Anterior cruciate ligament (ACL) injuries frequently occur in female athletes,1 and many undergo ACL reconstruction (ACLR) to return to sport (RTS).2 It has been reported that the incidence of a second ACL injury in the ACLR group was 0.47 per 1000 athlete exposures (AEs), which is about twice as high as the incidence of the initial ACL injury at 0.24 per 1000 AEs.3 Since secondary ACL injury is more common in females than in males,3 there is a relatively high need to investigate female athletes.
Seventy percent of ACL injuries occur via non-contact mechanisms4 and are caused by large ground reaction forces acting on the landing leg during rapid deceleration movements such as single-leg landing.5 These may not only result from the initial ACL injury, but also the risk of injury may be greater after ACLR. This is due to alterations in movement patterns, such as joint angles, joint moments, and coordination movements during single-leg landing and hopping post-ACLR, resulting from deficits in neuromuscular control.6,7 Afferent mechanosensory feedback control from the knee joint after initial contact (IC), which occurs to stabilize the joint during landing movements, takes more than 100 ms.8,9 However, ACL injury occurs 40 ms after IC,10 which renders feedback control too late to prevent ACL injury. Therefore, the timing of ACL injury may occur before feedback occurs. Consequently, assessing neuromuscular control before and after the onset of sensory input during single-leg landing movements can elucidate the ongoing neuromuscular activity strategies when an ACL injury is expected to occur.
In a previous study of biomechanics during drop-landing after ACLR, asymmetry of sagittal plane knee extension moment, knee valgus angle, and hip internal rotation moment were identified as risk factors for secondary ACL injury.11 Additionally, knee valgus angle enhances hip rotation moment and knee extension moment asymmetry, and deficits in single-leg balance are associated with the risk of reinjury. Knee abduction moment, internal tibial rotation moment, and anterior tibial shear force12 under axial impact produced clinically relevant ACL injuries.13 In summary, it has been pointed out that biomechanics during landing are an important factor in secondary ACL injury prevention after ACLR, but the changes in neuromuscular activity associated with movement patterns during landing are not clear. Therefore, it is necessary to examine these factors together to identify strategies for preventing secondary ACL injuries after ACLR.
The ability to minimize postural disturbances plays a crucial role in preventing injuries during sports activities.14 These efforts are achieved through compensatory and anticipatory strategies aimed at minimizing both unpredictable and predictable perturbations.8,15 Anticipatory postural adjustments (APAs) and compensatory postural adjustments (CPAs) come into play to maintain postural stability. APAs involve the activation or inhibition of lower limb muscles before internal or external balance perturbation occurs.16 Their primary role is to mitigate the impact of postural perturbation for maintaining balance.17 Conversely, CPAs are typically triggered by sensory feedback signals evoked by perturbation.9,18 Consequently, there is continuous muscle activity from the anticipatory to the compensatory phase during the movement.19 Therefore, it remains unclear during which phase of the landing ACL injury occurs in ACLR. By measuring APAs and CPAs before and after IC, it may be possible to clarify the mechanism of sensorimotor coordination during a risky landing motion. Also, it is possible that post-ACLR athletes’ neuromuscular control is affected by training during rehabilitation to return to sport.20 Hence, APAs and CPAs post-ACLR may exhibit distinct features compared to those observed in healthy subjects. However, the discrepancies between APAs and CPAs in female athletes with and without a history of ACLR have not yet been verified.
The purpose of this study was to clarify the differences in muscle activities of APAs and CPAs, lower limb kinematics, and kinetics between athletes with a history of ACLR and healthy athletes during single-leg landing. The hypotheses of this study were as follows: 1) muscle activity onset is delayed in the ACLR group compared to the control group; 2) muscle onset time was the same, but quadriceps activity during the compensatory phase was higher; and 3) hip adduction, internal rotation, and knee abduction angle at landing, along with hip adduction moment and knee abduction moment are reported to be higher in the ACLR group.21
METHODS
Participants
This cross-sectional study included nine female college athletes with a history of ACLR (ACLR group) and nine healthy subjects without a history of ACLR (CON group), selected from female athletes affiliated with university athletic clubs. The inclusion criteria for the ACLR group were as follows: 1) being 12 months post-surgery and having returned to full sports participation, 2) graft types being autograft (e.g., bone-patellar tendon-bone or hamstring tendon), 3) participation in sports activities such as cutting, pivoting, and jump-landing sports (handball, basketball, soccer, tennis, or lacrosse) prior to the injury, 4) no history of meniscal injury or surgery on either the ipsilateral or contralateral knee before ACL injury, and 5) absence of any disorders in the peripheral sensory system or history of surgery on the lumbar spine or lower limbs. The inclusion criteria for the CON group were: 1) no lower limb injuries and/or concussions within the prior three months and 2) no disorders in the peripheral sensory system or history of surgery on the lumbar spine or lower limbs.
The authors obtained written informed consent from all volunteers before their participation, following approval by the Ethics Committee of the Faculty of Health and Sports Sciences at the University (approval number 020-166).
Experimental task
The experimental task involved a single-leg landing from a 30 cm box, with participants instructed to land on the center of the force platform placed in front of the box. Additionally,
participants were instructed to place their hands on their waists to minimize the effect of balance retention by their upper limbs. For the single-leg landing motion, the injured leg was used in the ACLR group, while the dominant leg was used in the CON group. The dominant leg was defined as the leg preferred for kicking the ball by the participants. Throughout the experiment, participants were barefoot to eliminate the influence of footwear. Each participant was allowed to participate in several practice trials, and measurements continued until three successful trials were completed. Failed trials were characterized by foot slip after landing, loss of balance (where the sole of the opposite foot touched the floor or force plate), or inability to maintain the correct arm position.
Data collection
Surface electromyography (EMG) data were recorded at 1,000 Hz using a Data-LITE wireless EMG sensor (Biometrics Ltd.) and collected synchronously with the motion and force platform data. The skin at each electrode site was shaved and cleaned using an alcohol swab. Ultrasonography (Venue 50; GE Healthcare Japan) was used to define the anatomical properties of the superficial region of individual muscles for electrode placement, following the SENIAM recommendations.22 Electromyographic (EMG) activities of the tibialis anterior (TA), medial head of the gastrocnemius (MG), rectus femoris (RF), biceps femoris (BF), semitendinosus (ST), gluteus medius (GM), and adductor longus (AL) were measured. Electrodes were placed as follows: TA, 1/3 on the line between the tip of the fibula and the tip of the medial malleolus; MG, at the most prominent bulge of the muscle; RF, at 50% on the line from the anterior superior iliac spine to the superior part of the patella; BF, approximately halfway between the tibial lateral epicondyle and the ischial tuberosity over the muscle belly; ST, at 50% on the line between the ischial tuberosity and the medial epicondyle of the tibia; GM, 50% on the line from the iliac crest to the trochanter; and AL, between the sartorius and gracilis muscles.
A three-dimensional motion analysis system (VICON MX, Oxford, UK) captured task motions at a 250 Hz sampling rate using 13 infrared cameras. Thirty-five retroreflective markers were placed on the anatomical landmarks across each participant’s body according to the standard Plug-in Gait model (Genetic Hays marker set).23,24
Ground reaction force (GRF) data were measured using a force platform (Kistler Instruments, Inc., model 9281C, Winterthur, Switzerland). EMG and kinematic data were time-synchronized and acquired at 1,000 Hz. The GRF of the eight components (Fx, Fy, Fz, Mx, My, Mz, Cx, and Cy) were calculated for one force plate.
Data analysis
The following parameters were analyzed: (1) integrated electromyographic (IEMG) data, (2) muscle activity onset at the IC, (3) hip and knee joint angles, and (4) hip and knee joint moments. The analysis interval ranged from 150 ms before landing to 250 ms after landing, divided into the APA and CPA phases. Given previous studies suggesting that ACL injury may occur up to 100ms after IC, the analysis range was assumed to be no more than 100 ms after IC. IEMG was calculated for 4 different epochs, each lasting 100 ms in relation to T0 (defined as the instant when vertical ground reaction force exceeded 10N).25 APAs accounted for the electromechanical delay between muscle activity onset and torque generation, including the interval from T0 to 50 ms.26 The time windows for the four epochs were as follows: 1) from -150 to -50 ms (anticipatory activity, APA1); 2) -50 to +50 ms (anticipatory activity, APA2); 3) +50 to +150 ms (compensatory reactions, CPA1); and 4) + 150 to +250 ms (compensatory reactions, CPA2).
Anticipatory EMG activity was calculated as follows: EMG (APA1) represents the integral of EMG activity of the muscles from -150 to -50 ms with respect to t0, and similarly, EMG (APA2) is the integral of EMG activity, defined as the integral of the EMG signal from -50 to +50 ms with respect to t0. Compensatory EMG activity was calculated as follows: EMG (CPA1) represents the integral of the EMG activity of the muscles from +50 to +150 ms with respect to t0, while EMG (CPA2) was the integral of the EMG activity of the muscles from +150 to +250 ms with respect to t0.16
Data were analyzed offline using MATLAB (MathWorks Inc., USA). All EMG signals were rectified and filtered using a low-pass second-order Butterworth filter with a cutoff frequency of 20 Hz. For maximum voluntary contraction, IEMG data were calculated, and a 3 s IEMG was used to normalize the dynamic contraction recorded during landing (%MVC). Muscle activity onset was detected from the stance phase before landing for the baseline signal and 100 ms before approximately 300 ms from IC. Onset time was defined as the time when the EMG amplitude of the baseline signal exceeded the mean + 2SD for 12 ms.
Raw kinematic and kinetic data were filtered on the basis of frequency-content analysis of digitized coordinate data. GRF and marker trajectories were filtered at 15 Hz using a fourth-order Butterworth filter with VICON Nexus 2.0 software (Oxford Metrics Ltd., UK), consistent with previous studies.27 For all kinematic and kinetic data, the mean value of each 100 ms epoch was calculated. Joint angles and moments were averaged for each epoch, with time windows for the four epochs calculated at the same intervals as those for integral EMG.
Joint moments were computed on the reference leg side via a complete inverse-dynamic model implemented using the VICON Plug-in Gait. These moments were reported as external torques. Calculated GRF and joint moments were standardized according to the subject’s body mass (Nm/kg).
Statistical Analysis
To assess the normal distribution of the data, the Shapiro-Wilk tests were conducted prior to analysis. Independent-sample t-tests were used to ascertain significant differences in the onset time of muscle activity between participants in the ACLR and CON groups. For assessing significant interactions of each phase and participants in IEMG, hip, and knee joint angle, and moment, a two-way repeated measures analysis of variance (ANOVA) was utilized, followed by post hoc tests. Post-hoc Bonferroni multiple comparison procedures were used. Statistical analyses were performed using SPSS version 27 (IBM Corp., Armonk, NY, USA), with statistical significance set at p < 0.05. Additionally, a post-hoc power analysis was performed using G*Power software31(Version 3.1.9.6, University of Dusseldorf, Dusseldorf, DEU).28 The post-hoc power analysis, considering the significant result and the hip external rotation moment (effect size: 0.68), showed a power of 0.49 for both, calculated at an alpha level of 0.05.
RESULTS
Descriptive characteristics of participants are summarized in Table 1.
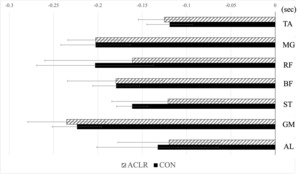
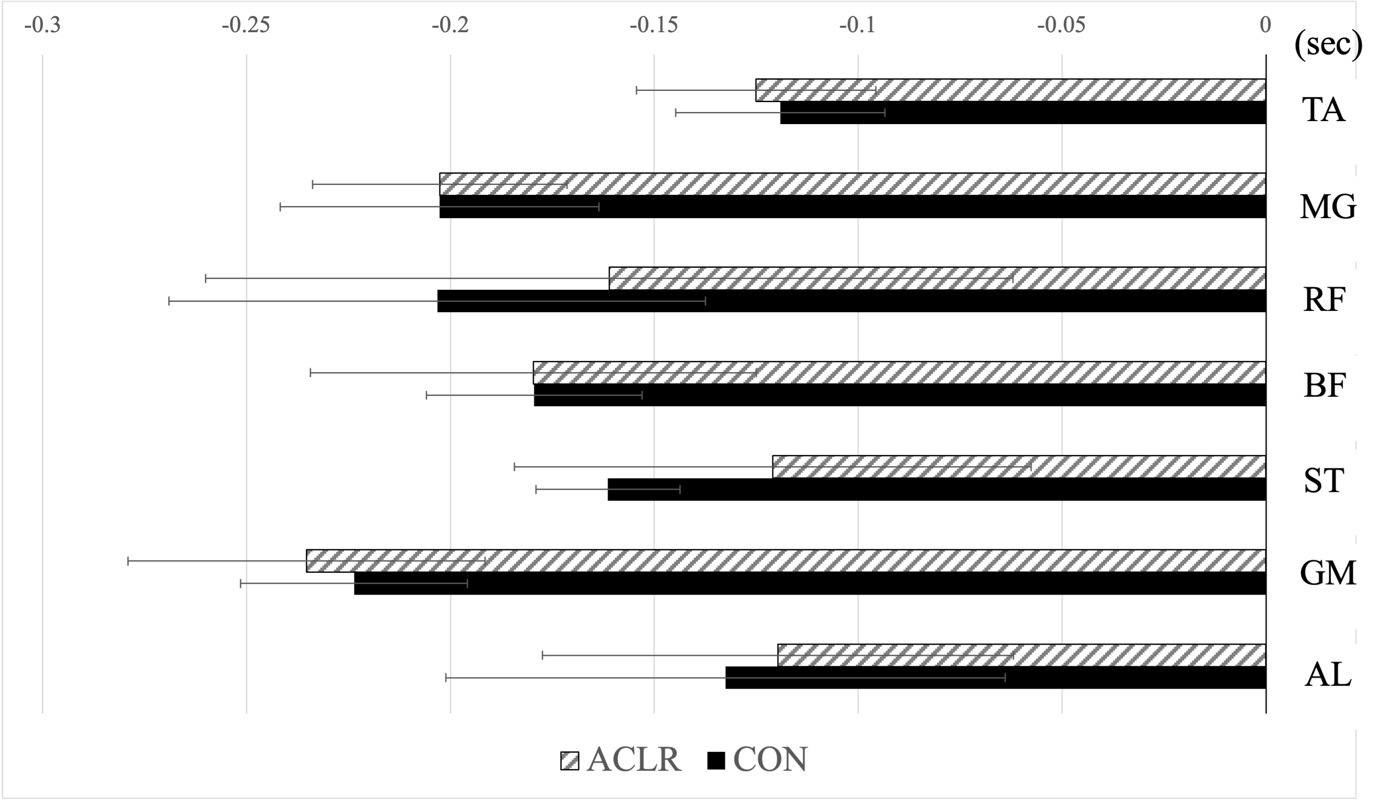
The onset times of muscle activity are depicted in Figure 1 for all participants, as well as specifically for the ACLR and CON groups. No significant differences were observed in the onset time of muscle activity (p > .05).
The IEMG results for each muscle in the ACLR and CON groups are presented in Table 2. Comparisons of the IEMG in each phase between ACLR and control groups revealed no interaction between groups (p>0.05) but significant main effects for the IEMG in each phase.
Multiple comparisons showed significantly higher values for CPA1 and 2 compared to APA1, and for CPA1 compared to APA2 in the TA (p < 0.05). In the MG, APA1 and 2 were significantly higher than CPA1 and 2 (p <0.05); in RF, CPA1 was significantly higher than APA1, and APA2 was significantly higher than CPA2 (p <.05); in the BF, APA1 and 2 and CPA1 were significantly higher than CPA2 (p <0.05); in ST, APA2 and CPA1 were significantly higher than APA1 (p <.05). In the GM, APA2 was significantly higher than APA1, CPA1and 2, (p <0.05). Also, in AL, APA2 and CPA1 were significantly higher than APA1 (p <0.05).
Comparison of hip and knee joint angles in each phase between ACLR and control groups showed no interaction but significant main effects for the joint angles in each phase (Table 3). Multiple comparisons revealed that the hip flexion angle was significantly higher in the following order: CPA2, CPA1, APA1, and APA2, (p <0.05). The angles of adduction and abduction were significantly greater in the CPA1 and 2 compared to APA1 and 2 (p <0.05). Similarly, the angles of internal and external rotation were significantly higher in CPA2 than in APA1, and in CPA1 and 2 compared to APA2 (p <0.05). The flexion angle of the knee joint increased significantly in the following order: CPA2 > CPA1 > APA1 > APA2 (p <0.05). Additionally, the angles of adduction and abduction were significantly higher in CPA1 than in APA1 and significantly higher in CPA2, CPA1, and APA2 (p <0.05). The internal and external rotation angles were significantly greater in CPA1 and 2 than in APA1 and 2 (p <0.05).
The hip external rotation moment exhibited a significant interaction between the ACLR and CON groups (p<0.018) (Table 4). Using the post hoc test, the hip external rotation moment of the ACLR group (CPA1=0.46 ± 0.16 Nm/km, CPA2 = 0.44 ± 0.1 Nm/km) at CPA1 and 2 was significantly larger than that of the CON group (CPA1 = 0.27 ± 0.06 Nm/km, CPA2 = 0.32 ± 0.09 Nm/km). For other parameters, a main effect was observed only for the phase, and multiple comparisons showed that the hip flexion moments were significantly higher for CPA1 and 2 compared to APA1 and 2 (p <0.05). The moments of adduction and abduction were significantly higher in the following order: CPA1, CPA2, APA2, and APA1 (p <0.05). APA1 and 2 exhibited significantly higher internal and external rotation moments than CPA1 and 2 (p <0.05). There was no interaction between the phase and joint moments of each group, and a main effect of the phase was observed only for the knee joint moments. Multiple comparisons revealed that the flexion moments were significantly higher in CPA1 and 2 compared to APA1 and 2 (p <0.05). The moments of adduction and abduction were significantly higher in the CPA1, CPA2, APA2, and APA1 groups (p <0.05). The moments of internal and external rotation were significantly higher in the following order: CPA1, CPA2, APA2, and APA1 (p <0.05).
DISCUSSION
This study aimed to elucidate differences in muscle activities during APAs and CPAs, as well as lower limb kinematics and kinetics between athletes with a history of ACLR and healthy athletes during single-leg landing. The hypotheses were: 1) muscle activity onset is delayed in the ACLR group compared to the control group; 2) muscle onset time is the same, but quadriceps activity in the compensatory phase is higher; and 3) hip adduction moment and knee abduction moment are higher in the ACLR group during landing. Contrary to these hypotheses, the results of this study revealed that female athletes who underwent RTS post-ACLR exhibited muscle activation patterns similar to those of healthy subjects in both anticipatory and compensatory phases of single-leg landings.
Onset times of muscle activity / IEMG
No significant differences were found in muscle activities during APAs and CPAs in any muscle between the ACLR and CON groups, which contradicts the study’s hypotheses. It has been suggested that factors such as muscle training29 and sensorimotor training included in rehabilitation may have contributed to early recovery of reflex excitability and motor neurons,30 enhancing neuromuscular control after ACLR.20 These may be adaptations due to learning by the central nervous system to plan anticipatory responses in advance.31 This finding aligns with previous research indicating that muscle activity in men after 14 months post-ACLR did not differ from that in healthy subjects.32 Furthermore, it suggests that female athletes who underwent RTS more than one year after ACLR may have recovered to a similar extent as healthy subjects in terms of muscle activation. Reduced muscle activity after RTS has been associated with poor performance and reduced dynamic stability of the knee during landing,33 underscoring the clinical significance of understanding changes in muscle activity at RTS.20,34 On the other hand, previous studies have reported earlier onset times or longer durations of muscle activities in the quadriceps and hamstring muscles before landing,35 indicating increased pre-muscle activity as a protective mechanism to stiffen the joint in preparation for impact after landing.36 However, these findings were mainly observed during the initial rehabilitation period (4 to 6 months post-surgery), whereas this study and others included subjects approximately 15 months32 and 60 months33 post-surgery. Thus, early EMG onset may only be evident during the initial rehabilitation period. These results emphasize the clinical importance of understanding changes in muscle activity during RTS.20,37 While the present study showed that factors leading to the risk of ACL injury by muscle activity onset were eliminated more than one year after ACLR, a reported secondary ACL injury rate of 9% in the involved leg within two years after ACLR3 suggests that other factors may still pose a risk for secondary ACL injury. ACLR has been associated with abnormal gamma loop activity, affecting the neuromuscular system through the spinal cord38 and sensory-motor deficits at the motor cortex level.39 Therefore, future research should explore changes in corticomotor excitability using functional MRI, transcranial magnetic stimulation, and H-reflexes to further understand these mechanisms.
Kinematics/Kinetics
The present study revealed a significant interaction in hip external rotation moments during the CPA phase, with higher values observed in the ACLR group compared to the CON group. However, no significant differences were found in lower limb joint angles between the two groups. Previous studies have shown that in kinematics, increased knee valgus and hip adduction angle during landing and cutting movements are risks for ACL injury,40,41 and decreased hip and knee flexion angles, along with increased GRF during such movements, can exert significant stress on the ACL.42 In kinetics, participants with smaller hip external rotator moments at the initial stage of landing were over eight times more likely to sustain a second ACL injury compared to those with greater hip external rotation moments.11 The similarity in lower limb joint angles between both groups in this study, alongside the increased hip external rotation moments in the ACLR group during CPAs, may be attributed to ACL injury prevention strategies implemented after ACLR, potentially affecting movement and postural control through kinetic changes. These strategies are considered ACLR-specific compensatory mechanisms aimed at reducing the angle of hip internal rotation occurring 50 ms post-IC, which corresponds to the feedback phase following sensory nerve input. Consequently, the high value of hip external rotation moment observed in this study may signify a postural strategy during single-leg landing movement after ACLR.
Limitations
This study had some limitations. First, lower limb muscle strength was not measured, which could have affected muscle activity during single-leg landing, especially considering reports indicating a relationship between muscle fiber conduction velocity and force in isometric contractions of the quadriceps during knee extension43 might cause a difference in muscle activity during single-leg landing. Second, the experiment was conducted in a laboratory setting and involved only single-leg landings, which may yield different responses compared to performance during actual sports activities. This context should be considered when interpreting the data. Third, the ACLR group comprised only female athletes who had returned to competition approximately one year or more after surgery, and it remained unclear when and to what extent muscle function had recovered. Therefore, longitudinal studies investigating ACLR muscle function and assessing training effects are warranted. Fourth, the study had a limited number of subjects, and only a post hoc power analysis was conducted. Despite the small sample size, meaningful results were obtained. Finally, this study only included the involved leg, although it is well-established that neuromuscular changes after ACL injury can also affect the contralateral leg.44 Including the contralateral leg in future studies will provide a better understanding of contralateral injury mechanisms and postural control after ACLR. In the future, it will be essential to examine the effects of post- ACLR status on muscle strength and surgical techniques.
Conclusion
The findings of the current study indicate that in female athletes who underwent RTS after ACLR, the onset of muscle activity during single-leg landing was similar to that of the control group. Both the amount and pattern of muscle activity during APAs and CPAs were similar to those observed in the control group. Additionally, no significant differences were observed in hip and knee joint angles between the two groups. However, during the CPA phase, the ACLR group exhibited higher values of hip external rotation moments compared to the control group. Clinically, this finding underscores the importance of assessing and addressing hip biomechanics and muscle function during rehabilitation to optimize movement quality and reduce the risk of secondary injuries. Future studies should investigate how variations in muscle strength affect neuromuscular control and landing mechanics, which could inform rehabilitation strategies aimed at optimizing strength and function.
Conflicts of Interest
The authors report no conflicts of interest.
Acknowledgments
The authors thank all the participants for their time and support. We would like to thank Editage (www.editage.jp) for English language editing.