INTRODUCTION
Anterior cruciate ligament (ACL) injury is a common sports injury with an incidence rate of up to 250 000 person-years.1 The risk of ACL injuries is significant among adolescent athletes, with approximately 1 injury per 10,000 exposures in female athletes, who are 1.5 times more likely than male athletes to suffer an ACL injury. A multisport female athlete has an approximately 10% risk of ACL injuries during her high school career. Sports such as soccer, basketball, lacrosse, and football pose a particular risk of ACL injuries in adolescents, highlighting the need for targeted interventions to optimize muscle strength and activation.2 ACL injury has long-terms consequences, reducing quality of life and increasing the risk of developing early knee osteoarthritis by sevenfold .3 The most common treatment to ACL injury is a surgical reconstruction of the ligament (ACLR), with nearly 130 000 patients operated on each year in the United States.4 There is evidence that only 55% of patients return to their previous level of sports performance after ACLR.5 Alteration in neuromuscular function is one hypothesis to explain this.6 Evidence shows that quadriceps strength deficit persist for up to two years after ACLR.7 After ACL rupture, there is knee joint effusion and therefore distension which is known to increase the recruitment of type II and type Ib afferent fibers, which have an inhibitory effect on the motoneuron.8 Pain, through the activation of type III and IV nociceptors is also known to inhibit the ability to contract the quadriceps.9 This interruption of afferent input leads to reflexive inhibition at the spinal level, known as arthrogenic muscle inhibition (AMI). AMI reduces both the availability of motor neuron (motor neuron pool)10 and the ability to voluntarily activation motor neurons to a normal extent (central activation failure). This motoneuron pool inhibition is seen at the spinal level. Although AMI typically occurs after ACL rupture, and persists for weeks after surgery, central activation failure has been shown to resolve by 6 months post-surgery and can delay effective progression of rehabilitation.10,11 Persisted AMI may cause strength deficits and, in combination with change in sensorimotor input due to the ligament rupture, causes neuroplastic changes within the motor cortex.12 Several studies show a reduced corticospinal excitability of the quadriceps in the long term after ACLR and return to sport (RTS).11,13 AMI is also thought to initiate quadriceps motor unit catabolism contributing to long term muscle atrophy and strength deficits.13 Quadriceps strength deficits after ACLR are impactful and it is associated with changes in locomotion biomechanics that may initiate the onset of osteoarthritis14,15 Failure to achieve a good level of quadriceps strength symmetry may increase the risk of re-injury.16 AMI has a potential impact on the long-term reduction in quality of life, function, and return to performance rate. Therefore, it is essential to develop an evidence-based treatment strategy for our patients. Our aim is to provide a clinical perspective based on the literature to review evidence regarding therapeutic intervention for AMI after ACLR.
ASSESSMENT AND CLINICAL SIGNS
Quadriceps levels of activation
The AMI phenomenon is often misunderstood leading to delays or difficulties in rehabilitation and recurrent injuries when returning to sport. Many physiotherapists believe that reaching full active knee extension or quadricipital contraction with superior patellar glide will put an end to the AMI and allow for earlier and more intensive muscle strengthening. This is a common misconception that can lead to knee pain, swelling, and inflammation if the load and modalities of strengthening are not appropriate. Scales that allow clinicians to quantify AMI and/or quadriceps contractility (AMI classification and Rachet Scale, respectively) exist. They suggest a classification to guide therapeutic intervention. A grade 0 is defined as normal contraction, grade 1: inhibited contraction with no extension deficit, grade 2 : inhibited contraction with associated knee extension deficit due to hamstrings’ contracture and grade 3 : passive chronic extension deficit due to posterior capsular retraction .17,18 However, these scales are primarily based on clinical experience, and there is limited evidence regarding their validity and reliability. Another concern is that these scales are based on vastus medialis activation only and not on the activity being performed. Many patients can lock their knee in an unloaded state (Rachet Scale)17 or even perform a straight leg raise (SLR) without lag (AMI classification),18 indicating minimal quadriceps activation (figure 1).19–21 However, adding resistance to exercises may cause a new flexion contracture to appear highlighting inadequate quadriceps activation during the proposed movement.
To prevent this, clinicians must be able to distinguish between three levels of activation (figure 1):
-
Minimal activation (SLR without lag with ability to sense quadriceps contraction )
-
Adequate activation (full knee extension regardless of activity). It is related to patients’ ability to perform a highly qualitative quadriceps contraction (including patient feedback and sensation control) during specific movement seem to be key indicators in quadriceps activation and strengthening after ACLR
-
Fast activation which is related to rate of force development (RFD) is important in the later phase of rehabilitation, particularly during the return to plyometric activities.22–25
There is a linear interdependent relationship between the three levels of activation. Clinicians should be able to classify patients within these three levels to propose an appropriate treatment strategy, regarding the clinical signs, that aligns with their goal of returning to sports.
Clinical signs
AMI is a complex neurophysiological phenomenon but it is important to understand the patient’s perspective. Clinicians need to understand that a patient may regain full extension without necessarily having full quadriceps activation. The evaluation of muscle activation may be a particularly important milestone for detecting the presence of AMI, as the persistence of this problem may hinder the return to sport and increase the risk of recurrence.
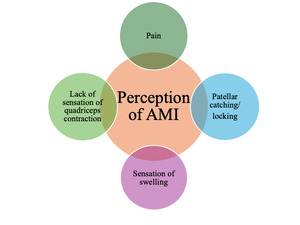
In the initial phase of rehabilitation, patients typically report experiencing pain spontaneously and during activity. Pain is commonly perceived on the medial and lateral aspects of the patella. Patients may not consciously perceive the contraction of the quadriceps and may omit it from their description. For instance, when performing exercises such as leg press/extensions or jumps, patients report experiencing painful sensations around the patella without any sensation of quadriceps engagement. Furthermore, patients frequently report experiencing patellar catching and locking, which may indicate impaired knee function (Figure 2).
REHABILITATION INTERVENTIONS (Table 1)
Targeting the muscle system
Various modalities may be considered in the treatment of AMI. Sonnery-Cottet et al. conducted a comprehensive analysis of studies highlighting the effectiveness of quadriceps-based interventions for AMI. Their review encompassed multiple studies, all of which underline significant improvements in quadriceps activation attributed to exercise therapy.26 The efficacy of various modalities, such as blood flow restriction (BFR) training has been shown to have superiority addressing muscle activity.27 BFR may be relevant in the early rehabilitation stage after surgery to prevent loss of muscle mass, strength, and function with lower load on the knee , helping to reduce the consequences of AMI.28,29 Reciprocal inhibition has also been studied, using hamstring fatigue to reduce quadriceps inhibition has shown some promising results, although based on limited quality evidence.30–32
Targeting the central nervous system
It is hypothesized that AMI induces changes in the motor cortex function. Various interventions have been tested with the objective of addressing these changes. For example, training the non-involved limb has been theorized to increase bilateral motor cortices (cross-education therapy (CET)) .30Investigations into CET have been conducted but yielded conflicting findings regarding its impact on quadriceps isometric strength.33–35 Motor imagery (MI) is another intervention that has been shown to increase excitability and activity of motor neuron within the motor cortex and the spinal cord through mental practice in healthy individual. Although, it is of great interest it has yet to be tested in individuals after ACLR .30
Virtual reality (VR) may create changes in cortical circuits.36 These adaptations can be produced by different VR systems. The simplest of these systems provide sensory feedback, while more immersive systems facilitate learning by placing patients in a motivating environment or by allowing them to learn based on the observation of reality, action, and/or imitation in a modified environment. The primary outcome is an improvement in therapeutic adherence. However, it is uncertain whether there is a significant improvement in the excitability of the motor cortex function, which would reestablish cortical drive to quadriceps motor neurons.30 EMG biofeedback is another modality that has gained increasingly popularity to address motor cortex dysfunction. Real-time biofeedback may help patient better voluntarily recruit specific muscle groups. Visual or auditory guidance are two components found in the literature used to help patient perceive muscle activation during exercise. This may be useful in lack of quadriceps activation due to AMI.37 Also when combined with conventional exercise biofeedback has been shown to be more effective in improving early quadriceps muscle activation than conventional exercise alone.38 Therefore, it could be useful in restoring quadriceps activation in the early stages of rehabilitation. It is also postulated that vibration may be beneficial in the treatment of deficiencies associated with AMI.. There is evidence that vibration is a strong stimulus to modulate the central nervous system through the activation of Ia afferents fibers that project on both the spinal cord and supra-spinal structures. When local vibration is acutely applied, a decrease in force-generation capacities has been reported, principally triggered by adaptations within the central nervous system. Thus, local vibration stimuli have the potential to act as a significant neuromuscular workload by inducing some fatigue which, when repeated, could enhancing voluntary recruitment and long-term neural adaptations leading to improved muscle function.30 Indeed, Souron et al shows that local vibration training can increase motor performance through a wide range of training and vibration parameters. While there seems to be a consensus about the neural nature of the induced adaptations, the precise mechanisms of these adaptations at both the spinal and supra-spinal levels remain to be clarified .39 Sonnery-Cottet et al revealed that vibration has low-quality evidence for efficacy to treat AMI.26 A randomized controlled trial looked at the effectiveness of whole-body vibration and the results are consistent with this evidence. Another randomized controlled trial was conducted to assess the efficacy of local vibration. Local vibration allows to allocate localized stimulus to one or a group of muscles in which vibration is believed to modify the excitability, associated with muscle contraction. Despite the lack of statistical power, the results were found to be promising for increased muscle strength, countermovement jump and sprint which is encouraging.40–42
Targeting the peripheral nervous system
Some of the modalities we have already mentioned may also be included in this section. Transcutaneous electrical nerve stimulation (TENS) and neuromuscular electrical stimulation (NEMS) are two modalities that have been studied to determine if they have any effect on AMI. It is theorized that sensory TENS aims to stimulate large-diameter sensory nerves and fibers (Aβ), which results in the presynaptic inhibition of pain signals to the transmission cell (T-cell), and thereby reduce the number of sensory pain signals being transmitted to the brain. Further, excitatory afferent stimuli created by sensory TENS may override the inhibitory signals, which can increase the excitability of the quadriceps motor neuron pool. Previous evidence demonstrates that TENS plays a significant role in managing joint pain.43 When applied before or during exercises, the decreased inhibition creates a window where potential for neuron motor excitability and motor unit is temporarily restored. NMES is a clinical modality that has the potential to prevent quadriceps activation failure and associated muscle atrophy. It initiates action potentials in intramuscular nerve branches, resulting in an unvoluntary contraction of the muscle. NMES exogenously stimulates the muscle, large diameter, type II muscle fibers which are thought to be selectively recruited, resulting in a greater potential for muscle force production.11 There is some evidence to support the efficacy of NMES and TENS for AMI following ACLR.26,35,44 Published by Michael J Toth & al,45 the first study addressing the usefulness of NMES showing a significant impact on muscle contractility. The second explored the effect of stimulation of the common peroneal nerve. This intervention shows a significant impact on reducing inhibition and increasing knee extension.45,46 Findings indicate that cryotherapy also demonstrates a degree of effectiveness in treating AMI.47–50 The application of cryotherapy to the knee joint have been shown to increases EMG activity and isometric strength in the quadriceps immediately after cryotherapy.51,52 Furthermore, compressive cryotherapy has been shown to have a small effect on swelling, pain and medication reduction compared to cryotherapy alone in reduce medical use in the first three days post ACLR.35 Cooling may also reduce the effect of AMI by altering sensory input from receptors in the skin or by reducing presence of pain.10
Conclusion
The management of AMI after ACLR is multifaceted and requires a comprehensive understanding of the various levels of activation, clinical signs, and potential modalities for interventions targeting muscles, brain, and nerves. It is crucial to recognize the complexity of AMI to avoid misconceptions and ensure appropriate rehabilitation strategies.
It is of paramount importance to evaluate quadriceps activation at different levels, including minimal activation, adequate activation, and rapid activation, to tailor rehabilitation programs to the individual patient’s needs and goals, through a shared decision process related to return to sports activities. It is of significant consequence to address patients’ complaints of pain, sensation of patellar catching, and impaired knee function to manage AMI effectively and facilitate successful rehabilitation outcomes.
To resolve any early signs of AMI we propose several muscle-based treatment modalities and AMI targeted interventions, including BFR, CE therapy, hamstring fatigue, and cryotherapy, that have demonstrated promising results in the treatment of AMI. However, further research is required to establish their efficacy conclusively. Similarly, cortical interventions, including virtual reality and transcranial magnetic stimulation, have demonstrated potential in modulating cortico-spinal circuits and improving therapeutic adherence. NEMS, TENS, and vibration therapy also seem promising in addressing AMI, and warrant further investigation in order to fully appreciate their effectiveness.
Overall, a comprehensive approach integrating multiple modality interventions targeting muscles, brain, and nerves may offer the most effective management of AMI following ACLR. Future research should focus on elucidating the mechanisms underlying AMI and evaluating the long-term effectiveness of various already existing interventions to optimize functional outcomes and improve patients’ quality of life and return-to-performance rates and delays.