INTRODUCTION
With an incidence of up to 25 cases per 100,000 person-years, the shoulder is one of the most frequently dislocated joints in the body.1,2 Historically, first time dislocators had been managed conservatively, yet recent authors suggest that high risk patients benefit from early surgical intervention, as they have observed a decrease in recurrence rates and an increase in return to competitive activities.3–6 The addition of the remplissage procedure, which involves an infraspinatus tenodesis and posterior capsulodesis to an arthroscopic Bankart repair, in patients presenting with a Hill-Sachs defect, further decreases recurrence rates in this patient population.7–12
Since at least 65-70% and up to 100% of the first time and recurrent anterior shoulder dislocators, respectively, present with a Hill-Sachs defect, arthroscopic Bankart plus remplissage (ABR) has gained popularity in recent years, however, there has been some concern raised regarding potentially compromising shoulder range of motion.2,7,13–16 Therefore, post-operative rehabilitation plays an important role in both addressing and optimizing range of motion as well as facilitating optimal healing of the of the posterior structures following the addition of the remplissage procedure.17,18
The purpose of this systematic review was to examine clinical studies that described a post-operative rehabilitation protocol after an ABR procedure. It was hypothesized that the present study would find substantial variability among the included studies and that, among the studies, there would be little to no distinct regimen from that used for rehabilitation of isolated Bankart repair.
MATERIALS AND METHODS
Search strategy
The present study was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.19 Clinical studies discussing outcomes after an arthroscopic Bankart repair augmented by a remplissage procedure for either Bankart or Hill-Sachs lesions of the shoulder were identified by searching Medline / PubMed (National Library of Medicine, NCBI), Embase (Elsevier, embase.com), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL Complete, EBSCOhost) on July 10, 2023. Controlled vocabulary terms including “Bankart lesion”, “labral tear”, and “remplissage” were utilized and combined via Boolean operators. The search strategies were designed and carried out by a health sciences librarian. No publication date limits were applied. The exact search terms used for each of the databases are provided in the Appendix 1.
Inclusion and exclusion criteria were determined prior to running the searches. Studies that evaluated patients after arthroscopic stabilization for unidirectional anterior glenohumeral instability with the addition of the remplissage procedure, with no other concomitant procedures performed (rotator cuff repair, ALPSA repair, HAGL repair) except for a SLAP/long head of the biceps repair, written in English, and at least one year minimum follow up were included. Review articles, systematic reviews, abstracts, case reports, technical notes, cadaveric or biomechanical studies, and non-English language studies were excluded. Additionally, articles were excluded if minimum follow up was < 1 year, if only revision cases were included, and if overlapping cohorts were found among studies.
Data Collection
Search results were assessed for eligibility by two independent reviewers who performed both abstract screening and full text review. Data of included studies was extracted by both reviewers using a pre-designed extraction form on Microsoft Excel spreadsheet (Version 2007, Microsoft, Redmond, WA, USA). Upon discrepancies, the issue was addressed by a third reviewer and resolved by consensus.
Extracted data included title, authors, year of publication, study design, level of evidence, number of subjects per included study, mean age, % of females, mean follow-up, % glenoid bone loss, size of the Hill-Sachs lesion, and whether the Hill-Sachs lesion was engaging or not. Additionally, the rehabilitation protocols of each included study were assessed, and data grouped and stratified by type and duration of immobilization, exercises allowed in the early post-operative period, early range of motion restriction, start of passive, active assisted, and active exercises, time to achieve full range of motion, start of formal strength training, targeted muscle strengthening plan, return to sport criteria (including time), and whether the rehabilitation protocol differed between performed procedures.
Study Evaluation/Quality Assessment
All non-randomized studies were assessed for quality and risk of bias using the Methodological Index for Non-randomized Studies (MINORS) instrument where comparative studies may reach a global score of 24, whereas non comparative studies may add up to a maximum of 16 points.20 Additionally, comparative/cohort studies were also assessed by the Newcastle-Ottawa Scale for cohort studies.21 Finally, the only clinical randomized controlled trial (RCT) included in the current study was assessed for quality and bias using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) tool for RCTs.22
Statistical Analysis
Descriptive statistics including means, proportions, and ranges were calculated and presented using Microsoft Excel (Version 2007, Microsoft, Redmond, WA, USA). Due to the highly heterogeneous nature of the reported data, no quantitative analysis was conducted; therefore, the data is presented qualitatively.
RESULTS
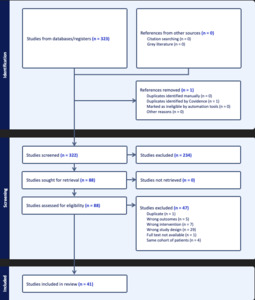
The search strategy yielded 550 studies across all three databases, after removal of duplicates, 322 were available for screening using Covidence software (Veritas Health Innovation, Melbourne, Australia). The PRISMA Flow Chart Diagram is presented in Figure 1. After screening and full text review, data were retrieved from 41 included studies, including one Level I,11 two Level II,15,23 24 Level III,12,24–46 and 14 Level IV7,47–59 studies. A total of 1,307 patients were included presenting with a mean age of 27.5 years (range 14-72). Among the included patients, 18% were female and all had a minimum follow up of at least 12 months (range 12-180 months) after undergoing an ABR procedure. All patients had <30% glenoid bone loss and a range of 10-50% of the humeral head size, Hill-Sachs defect. Additionally, 37 studies included engaging/off-track lesions7,11,23–44,46,48–59 whereas two studies reported only on non-engaging/on-track lesions12,45 in their cohort with two other studies not reporting on the engagement status of the included population.15,47 A summary of patient demographics can be found in Table 1.
Study Evaluation/Quality Assessment
The average MINORS grade for non-comparative studies was 12.6 (range 11-15), whereas for comparative studies, the mean MINORS grade was 20.6 (range 16-24). Additionally, all comparative studies fell under the “Good Quality” category of the Newcastle-Ottawa scale. Finally, the GRADE tool assessment of the only Level 1 study,11 was classified as “High quality.” The quality scores for each individual study can be found in Table 2.
Physical Therapy Protocols
A summary of the rehabilitation protocols of all included studies is shown in Appendix 2. All 41 studies7,11,12,15,23–59 reported on the type of immobilization where the most common reported type of immobilization was a simple sling utilized in 34 of the studies11,12,15,23,24,26–35,37,38,41–43,45–57,59 (83%). Four studies36,39,40,44 (9.7%) reported the usage of either an abduction or neutral rotation brace, in contrast to the rehabilitation protocol of two studies25,58 (4.8%) which used a shoulder or external rotation immobilizer. One additional study7 (2.4%) reported the usage of an arm pouch as the type of immobilization used in their rehabilitation protocol. In terms of position of immobilization, a sling in neutral rotation was employed in 20 studies11,15,29,30,32–35,37,41,43,45,46,48,51,53,54,56,57,59 (48.7%), and the remaining 21 studies7,12,23–28,31,36,38–40,42,44,47,49,50,52,55,58 (51.3%) included a wide range of 11 different immobilization positions.
Forty studies7,11,12,15,23–53,55–59 (97.5%) reported on time of immobilization after surgery which ranged from three to six weeks. Out of the initial 40 studies, eighteen23,26–30,32–35,42,44,45,49–51,58,59 (43.9%) immobilized the operated shoulder for six weeks, with this time point being the most frequently reported time of immobilization followed by four weeks reported in nine studies12,24,40,41,43,46,48,53,55 (21.9%). Upon stratification by level of evidence, the mean immobilization time was 4, 4.9, and 4.7 weeks for Level I/II, III, and IV studies, respectively.
Allowance of post-operative motion, described in 17 studies7,11,12,23,26,34,36,38,42,44,46,48–50,53,54,58 (41.4%), was also an important extracted data point in the current review. Among the included studies, either pendulum or isometric exercises were allowed with the specific time of initiation ranging from post-operative day one to four weeks after surgery. Pendular and/or isometric exercises starting on post-operative day one were the most common indication regarding post-operative motion allowance, as it was reported in seven of the 17 included studies11,38,46,48–50,58 (41.1%).
Twenty-three studies11,12,15,23–28,30,35,41–44,49–54,57,58 (56%) controlled for range of motion restriction in their rehabilitation protocol with four-to-eight-week post-operative ER restriction being the most reported15,25,42,43,49,50,54,58 (n=8 studies/34.7%). Other studies restricted abduction and external rotation11,23,26,30,52,57 (n=6 studies/26%), forward flexion and external rotation12,27,35,44,51,53 (n=6 studies/26%), forward flexion past 90 degrees24,28 (n=2 studies/8.6%), and forward flexion plus external rotation at side and in abduction41 (n=1 study/4.3%). The mean time for range of motion restriction in any plane of motion stratified by level of evidence was 7.2, 5.7, and 5.6 weeks for level I/II, III, and IV, respectively.
Thirty-one studies7,11,15,23,25,26,30,31,34,36–46,48–52,54–59 (75%) described at least one of the following in their rehabilitation protocol: start of self, passive or active-assisted motion and the start of formal physical therapy. Initiation of either active-assisted or active motion was the most reported instruction with the initiation time ranging from two to twelve weeks after surgery. Additionally, only four Level III studies12,26,29,41 (9.7%) out of the initial 41, described the goal, in weeks, by when patients must have achieved full range of motion.
Twenty-nine articles7,11,12,23,26–29,31–43,46,50,52,53,56–59 (70.7%) reported on the starting point for shoulder specific strength training initiation with an average starting date of 12, 9.8, and 11.7 weeks for Level I/II, III, and IV, respectively. Moreover, 24 studies7,11,12,23,28,31,34,35,37–43,45,46,50,52,53,56–59 (58.5%) made further suggestions regarding the type of targeted strengthening, yet no detailed targeted strengthening regimen was described in any of the included studies.
Thirty-seven7,11,12,23,24,26–35,37–46,48–59 (90.2%) studies reported on the time to return to sport/play (RTS/RTP) with a mean time of 5.2 months for level I/II, 6.4 months for Level III, and 6.4 months for Level IV studies. Nonetheless, only 11 studies7,12,31,34–36,41,42,46,53,57 (26.8%) used objective based criteria (strength, ROM, pain, stability) to assess for return to sport/play.
Finally, for comparative studies, 3/3 (100%) of the included Level I/II11,15,23 and 17/19 (89.4%) of the included Level III studies12,24–26,28–32,34–36,39,41,44–46 (24 total Level III studies, however five were not compared against other type of procedure) reported using the same rehabilitation protocol for their comparative counterpart regardless of the procedure performed. The protocols utilized in these studies did not adopt an individualized approach based on the type of procedure performed.
DISCUSSION
The variability in reported rehabilitation protocols following ABR for anterior shoulder instability is the most notable finding. Hence, the present review will discuss each aspect of these protocols, encompassing immobilization strategies, early motion exercises, movement restrictions, initiation of formal physical therapy, strength training, and the timeline for returning to sport by delving into the justifications presented in articles, the resultant outcomes reported in these studies, and juxtaposing these findings with our institutional rehabilitation protocol. Through this comprehensive exploration, the authors aim to describe the intricacies surrounding post-operative care, unravel various complex aspects that contribute to the diversity of rehabilitation treatments, identify potential opportunities for refining the management of patients undergoing this combined surgical intervention, and provide the authors’ suggested rehabilitation protocol based on the literature and the clinical expertise of the senior authors.
Among the key findings, it is evident that the type and duration of immobilization exhibit considerable diversity across the included studies. Notably, the majority of studies (83%) favored the utilization of a sling as the primary mode of immobilization. However, intriguingly, a subset of studies (17%) opted for alternatives such as abduction or neutral rotation braces, with a few even employing an arm pouch for immobilization. This observed divergence in immobilization approaches underscores the lack of standardized consensus in the field, possibly influenced by surgeon preference, equipment availability, patient characteristics, or perceived benefits. Moreover, the position for immobilization was discussed at length within the retrieved articles with at least a half reporting immobilization in neutral rotation whereas the other half reported a variety of different positions. Gaunt et al.,60 in the American Society of Shoulder and Elbow Therapists´ (ASSET) rehabilitation guideline for arthroscopic Bankart repair, suggest immobilizing the shoulder with a sling in neutral rotation for patients who have undergone the aforementioned procedure. Yin et al.61 evaluated the position of immobilization after an arthroscopic shoulder stabilization procedure. In their study, they described outcomes after immobilizing the shoulder in external rotation. Although no control group was included in their study, their results showed that immobilization in external rotation (ER) was associated with full range of motion recovery at 3 months, low risk of recurrence in the first 12 months, high functional outcomes scores, and low VAS pain scores. Minkus et al.62 showed no functional or range of motion differences after immobilization in either internal or external rotation after an arthroscopic anterior shoulder stabilization procedure. However, it is not known whether these results could translate after the addition of the remplissage procedure as, to the authors knowledge, no study to date has compared various positions of shoulder immobilization, in aims of enhancing the healing of the posterior structures, after arthroscopic stabilization with the remplissage procedure.
Regarding the duration of immobilization, a range of three to six weeks emerged from the synthesis of the studies. The consensus around a six-week immobilization period is evident, with this timeframe being the most frequently reported among the included studies. However, the mean immobilization time for Level I and II studies averaged four weeks, potentially reflecting a trend toward shorter immobilization periods in higher-quality studies. This discrepancy in immobilization duration raises questions regarding the balance between maintaining shoulder stability through a prolonged immobilization period and the potential benefits of early mobilization in terms of mitigating muscle atrophy and stiffness. Kim et al.,63 reported no differences in terms of recurrence rates after either immediate mobilization or immobilization for at least three weeks. However, their RCT only included patients who had undergone an arthroscopic Bankart repair alone, therefore, no conclusion can be made for cases where the remplissage procedure is added. Longer immobilization times may be warranted upon the addition of the remplissage as tendon-bone healing, from reattachment of the infraspinatus tendon into the bone divot, is expected to occur.60 On the other hand, due to concerns regarding loss of motion following the remplissage procedure, there could conceivably be advantages to earlier mobilization as well.
Early post-operative range of motion exercises emerged as another focal point of disparity. While pendulum and isometric exercises were widely incorporated into rehabilitation regimens, the time of their initiation varied significantly, ranging from the immediate post-operative period to four weeks after surgery. Among the diverse early motion strategies, the prominence of pendulum exercises initiated as early as postoperative day one highlights the growing acceptance of the benefits of initiating controlled movement earlier in order to potentially speed up the recovery process and reduce complications that may arise from prolonged immobilization. In the only available rehabilitation guideline for arthroscopic Bankart repair,60 only isometrics with the arm adducted to side in neutral rotation are allowed in the first post-operative weeks while MacDonald et al.,11 in the only included RCT, allowed patients from both groups (isolated Bankart repair or ABR) to perform pendular exercises for the first three weeks post-surgery. Substantial variation exists within the rehabilitation protocols after an isolated arthroscopic Bankart repair, as it has been previously reported.64–66
Notably, restrictions on specific shoulder movements during the early postoperative period demonstrated marked heterogeneity. As established by Gaunt et al.,60 controlling for range of motion is of extreme importance as unrestricted movement could stress the repair past the healing stimulatory threshold causing failure. While the majority of studies included in the present systematic review advocated for limitations on external rotation for four to eight weeks, others imposed constraints on abduction and external rotation, or forward flexion beyond 90 degrees. This diversity in movement restrictions can be attributed to the absence of a widely accepted approach to balance the need for protective immobilization against the benefits of graded early motion. For an isolated Bankart repair, it has been suggested that range of motion not to exceed 30 degrees of external rotation with the arm in adduction would be a safe boundary for the repair, yet whether this remains safe upon the addition of the remplissage is unknown.60
The initiation and progression of formal physical therapy represented an additional realm of variability. Although the most commonly reported instructions involved the initiation of active assisted or active motion exercises, the start times ranged widely from two to 12 weeks after surgery. As established in ASSETs rehabilitation guideline, gradually progressing through degrees of range of motion is of utmost importance as reports suggest an inverse correlation between the integrity of the repair and the speed of regaining range of motion.60
Remarkably, the absence of detailed, targeted strengthening regimens in any of the included studies is a noteworthy finding. Despite the emphasis on initiating strength training concomitantly while achieving full motion, the lack of standardized protocols raises concerns about the optimization of muscle recovery and function, which are pivotal for patients seeking to return to sports or daily activities.
Return to sports timeline is a multifaceted parameter. The mean time to return to sport/play ranged from 5.2 to 6.4 months across different study levels. This variation could potentially be attributed to factors such as study design, patient selection, and differing definitions of “return to sport.” Currently, subjective criteria plus time are the most widely used criteria to assess for readiness to return to sport.1 Nonetheless, recent studies have reported on the need of shifting towards an objectively based criteria system, as it has been shown to lower recurrence rates.60,67,68 However, based on the results of this review and from previously published studies, marked heterogeneity exists and the absence of reporting on usage of objectively based and functional criteria to clear a patient to return to their sporting activity is problematic.67 In the present review only 11 studies used previously defined criteria (strength, ROM, pain, stability) to assess for return to sport/play. Additionally, in an international consensus statement, the evaluation of psychological readiness to return to sport after anterior shoulder instability reached unanimous consensus.69 The usage of the Shoulder Instability Return to Sport after Injury (SIRSI) scale can be used for assessment of the psychological readiness to return to sport.70 In this review no studies took psychological readiness into account for readiness to return to sport assessment. Moreover, in a survey of shoulder surgeons evaluating the criteria used for clearance to return to sport, 92% of the participants stated that the addition of the remplissage procedure to an arthroscopic Bankart did not influence on the physician’s decision to clear athletes to go back to their activities. In the authors´ experience, patients undergoing an additional remplissage procedure must have an individualized approach which may differ from patients undergoing an isolated arthroscopic Bankart repair as the addition of the infraspinatus tenodesis and capsulodesis has been shown to potentially result in diminished motion and strength at six months, when compared to its isolated counterpart.16
The trend observed in comparative studies reveals a remarkable homogeneity in the rehabilitation protocols adopted, irrespective of the specific procedure performed. All Level I/II studies (100%) and a substantial majority of Level III studies (89.4%) adhered to identical rehabilitation regimens for their comparative counterparts, regardless of whether arthroscopic Bankart repair was combined with remplissage or if any other procedure was performed (Latarjet, autografts). This notable lack of tailoring rehabilitation protocols based on the distinct surgical interventions raises concerns about the optimization of post-operative care. The emphasis on a uniform rehabilitation strategy could inadvertently limit the potential benefits that tailored protocols might offer, disregarding the varied biomechanical alterations and recovery trajectories introduced by the combined surgical procedures.
A limitation of the present study includes the observed variability in the vocabulary used to describe aspects of the rehabilitation protocols which predisposes the readers to confusion (i.e. whether shoulder immobilizer also refers to sling, strengthening terminology, etc.). Moreover, some of the included studies did not clearly state the physical therapy rehabilitation protocol, with some describing their followed protocol in two or three sentences. Additionally, although three studies were Level of evidence I or II, the remaining 38 were Level of evidence III or IV which reflects the low-quality of some of the included studies. Lastly, specific soft-tissue injury magnitude was not consistently reported within studies, which could have potentially contributed to the decision of early versus delayed range of motion initiation seen across studies.
AUTHOR’S PREFERED TECHNIQUE
With the considerable lack of consensus on the most appropriate rehabilitation progression following an ABR, the authors feel it important to provide an example protocol for clinicians to follow (Appendix 3). It is worthy to mention that the proposed approach has not been subjected to rigorous study or scrutiny and is merely a proposed exemplary protocol.
Considering what is known about the surgical technique and healing constraints of the involved tissues, the authors’ approach is to progress patients similar to an isolated Bankart repair with small adjustments to progression of internal rotation range of motion and the initiation of external rotation strengthening. This is designed to protect the remplissage procedure, while also avoiding the loss of motion or persistent loss of function.
Patients are immobilized in a simple sling by their side for the first four weeks, though are allowed to start immediate physical therapy. It is the author´s preference to have a slow and gradual restoration of range of motion, rather than delay for too long and fight hypomobility. Range of motion is slowly restored over the first 8-10 weeks, similar to after a Bankart repair. While internal rotation is initiated early with gentle pain-free range of motion, caution to not push through discomfort for the first eight weeks is important. Also, regarding strengthening, avoidance of external rotation isometrics and delaying the initiation of light isotonic exercises is the suggested until week six.
In contrast to the knee, objective functional return to sport tests after shoulder instability procedures have not been commonly standardized. However, functional tests for the upper extremity do exist and should be considered and the authors believe that further research should be performed prior to definitively recommending any particular battery of tests.
The following criteria could help guide the decision in the interim: absence of pain/tenderness, at least six months after the procedure (allows repaired structures to fully heal), achievement of full functional active range of motion, objectively measured injured shoulder strength (at least 90% limb symmetry index [LSI]) including ER and IR at 0º and 90º of abduction, performance of the Closed Kinetic Chain Upper Extremity Stability test (+/- open chain testing for overhead athletes),and psychological readiness to return to sport measured via the SIRSI scale.
Based on the authors´ collective experience and understanding of the basic science and healing of the repair, it is believed that this progression is safe and effective at restoring function without disrupting the natural healing process. Future studies should be conducted to prospectively evaluate this approach and to identify the optimal rehabilitation protocol while incorporating appropriate functional tests for guidance of return to sport. It is the author’s belief that following these strategies after remplissage augmentation could maximize function while limiting stiffness and recurrent instability.
CONCLUSION
The results of the present systematic review expose the variability among rehabilitation protocols following ABR. This variability prompts consideration of the underlying factors influencing these disparities and underscores the need for future research to elucidate optimal rehabilitation. Based on results and the senior authors´ clinical experience, a suggested rehabilitation approach is provided, similar to an isolated Bankart repair, with additional precautions surrounding internal rotation range of motion and external rotation strengthening.
Conflicts of interest
The authors report no conflicts of interest.