INTRODUCTION
Musculoskeletal injuries, accompanied by acute or chronic pain are prevalent health issues that can lead to significant disability. Although musculoskeletal pain prevalence is high, its treatment is often inadequate. Untreated acute pain can evolve into chronic pain due to prolonged nociceptor activation, neuronal changes, and central sensitization of spinal neurons.1,2 Nonpharmacological approaches aim to alleviate pain, reduce edema, and expedite muscle recovery, with heat or cold therapy frequently utilized.3
Cryotherapy, from the Greek words “kryos” meaning “cold” and “therapeia” meaning “healing,” has been a pivotal therapeutic intervention for several decades. This modality has found broad applicability across diverse medical disciplines including physiotherapy, sports medicine, and even surgical aftercare. Traditionally employed for pain management and to treat a range of musculoskeletal injuries, cryotherapy has evolved to have a multitude of clinical applications, offering symptomatic relief, modulation of edema and potentially accelerating the healing process.4
Although the therapeutic utilization of cold treatment spans various traditional medicine practices, its systematized clinical application, especially in the domains of sports medicine and physiotherapy, has been more prominent over the last few decades. Recent advances have honed the technique, optimizing its benefits while minimizing risks.5
Cryotherapy has shown promising outcomes in various clinical scenarios, especially in treating conditions like spasticity, joint restriction in rheumatic diseases, and certain types of pain. These therapeutic effects stem from the physiological responses to cold, including changes in muscle tension, reflex activity, vascular effects, and nerve transmission. The utility of cryotherapy is further underscored by its success in managing symptoms of conditions like multiple sclerosis, craniocerebral injuries, and arthritis.6 Furthermore, cold application has been shown to have neuromuscular benefits, reducing muscle spasm and improving muscle function.7 Cryotherapy, applied via ice packs, cold water immersion, and cryotherapy chambers, is commonly deployed for the treatment of both acute and chronic musculoskeletal conditions. These range from post-operative swelling to sports injuries, including sprains, strains, and contusions. There are absolute contraindications against the use of cryotherapy like sensitivity to cold, specific diseases like Raynaud’s and cold urticaria, certain medication effects, technical device defects, and others. Hemodynamic and spirometric evaluations further identified contraindications related to the cardiovascular and respiratory systems, such as aortic valve defects and specific heart diseases. Finally, there are relative contraindications, including age over 65 and a history of venous blood clots. A comprehensive pre-treatment assessment, including detailed medical history review, physical examination, and hemodynamic and spirometric evaluations, ensure the safe and effective application of cryotherapy for each patient.8
A diverse array of cooling agents is available, each of these cooling agents has specific advantages and disadvantages influencing their selection for treatment. Ice packs are effective in reducing pain 24 to 72 hours after childbirth compared to no treatment9,10 and in treating acute sports injuries by reducing pain and recovery time.11 However, they may cause discomfort and require additional analgesia in some cases,9,10 and are less effective in reducing vaccination pain in pediatric patients.12 Vapocoolant sprays are effective in reducing pain during vaccination in adults and some pediatric cases,12 and are preferred over ice packs for intravenous catheter placement,13 but may cause mild discomfort during application.14 Cooling gels are user-friendly and comparable to ice packs in efficacy for treating acute ankle sprains,15 but there is limited evidence on their superiority over traditional ice packs.9,10 Therefore, the choice of cooling agent depends on the specific injury or condition, patient preferences, and cost considerations.
Fisiocrem® spray is a vapocoolant spray for topical application that is administered directly onto the skin and contains a blend of butane, propane, isobutane, ethyl alcohol, and natural aromatics. According to preliminary results from a physical analysis of the spray’s cooling effects, a single application demonstrated significant reduction in skin temperature and local blood flow, which is expected to help alleviate pain and reduce swelling in cases of muscular injuries.16
The primary aim of this descriptive observational cohort study was to assess both immediate and sustained pain relief in subjects with mild to moderate musculoskeletal complaints. The hope is that the results will contribute to a gap in current scientific regarding the effectiveness of treatments for mild to moderate musculoskeletal pain.
METHODS
The study was conducted at the Palacio de Deportes de Torrevieja, Alicante, Spain sports resource and involved 60 amateur football players with knee or ankle injuries who volunteered to participate and provided informed consent. One participant withdrew, leaving 59 who completed the study. Ethical considerations were meticulously addressed; informed consent was obtained from all participants prior to the initiation of any study-related procedures. The study protocol was approved by the Comité Ético de Investigación con Medicamentos (CEIm) Regional de la Comunidad de Madrid, Spain.
Eligibility criteria for the study required participants to be over 18 years of age, recruited from the Sports Center. Acute pain cases were selected from athletes experiencing sports injuries during training or competition, while chronic pain cases included amateur athletes undergoing therapy or rehabilitation. Participants needed to exhibit mild to moderate musculoskeletal pain, whether joint or muscle-related, defined as a Visual Analogue Scale (VAS) score between 3 and 6. Participants with chronic neuropathic pain, fibromyalgia, neoplasia, known allergies to any component of the product, severe acute injuries, impaired skin integrity, or pregnancy were excluded from the study.
The trial had a duration of three weeks per patient. Each participant made three visits to the center for assessments throughout the study’s duration, thereby ensuring a comprehensive longitudinal evaluation of both immediate and sustained effects of the treatment. Follow-up occurred one week after the last administration of the treatment to assess any long-term effects or potential adverse reactions. Pain intensity was measured by a Visual Analogue Scale (VAS) from 0 – no pain to 10 - maximum pain, Joint range of motion (ROM) assessments were conducted at each specified time interval throughout the study, including T-1 (before treatment), T0 (beginning of treatment), T2-min, T5-min, T10-min, T15-min, T30-min, T60-min, T7-days, T14-days, while participants were actively receiving treatment exclusively with the cryotherapy spray, and T21-days (one week after treatment cessation). Participants were advised to refrain from sports practice while maintaining their normal daily movement patterns during treatment. Time intervals were selected to capture both immediate and sustained effects of the intervention, allowing for comprehensive monitoring of changes in pain and mobility over time.
Outcome measures
Pain intensity was evaluated using the Visual Analogue Scale (VAS), a widely accepted tool with proven reliability and validity for musculoskeletal pain measurement (reference needed for VAS reliability and validity). Participants recorded their pain level by marking a point along a 100 mm horizontal line on paper, ranging from 0 (no pain) to 100 (worst pain imaginable). Both active and passive knee or ankle joint range of motion (ROM) were assessed using a goniometer, always performed by the same qualified physician trained in ROM assessment to ensure accuracy. Because two different joints (knee and ankle) were studied, the scores were grouped into four categories: 1 - Severe limitation, 2 - Moderate limitation, 3 - Mild limitation, and 4 - Normal ROM. For the knee (normal ROM: flexion 0-135°, extension 0°), the categories were: Severe limitation (flexion 0-45°, extension >15° flexion), Moderate limitation (flexion 46-90°, extension 5-15° flexion), Mild limitation (flexion 91-120°, extension 1-5° flexion), and Normal ROM (flexion 121-135°, extension 0°). For the ankle (normal ROM: dorsiflexion 0-20°, plantarflexion 0-45°), the categories were: Severe limitation (dorsiflexion 0-5°, plantarflexion 0-15°), Moderate limitation (dorsiflexion 6-10°, plantarflexion 16-30°), Mild limitation (dorsiflexion 11-15°, plantarflexion 31-40°), and Normal ROM (dorsiflexion 16-20°, plantarflexion 41-45°).
Clinical safety was comprehensively assessed through meticulous recording of adverse events to evaluate the tolerance of the product and any potential adverse reactions. Secondary objectives included assessing the immediate cooling effect following the initial application of the product. This effect was measured using a subjective Likert scale, where participants rated the cooling sensation on a scale from 1 (‘no cooling effect’) to 5 (‘very strong cooling effect’). Additionally, subjective evaluations were collected regarding the time to symptom improvement or relief, the extent of mobility in the affected area, and the overall tolerance of the product. See Appendix 1 for survey variables/questions.
Participants’ subjective experiences were evaluated using custom-designed questionnaires developed by the research team based on existing validated pain and mobility assessment tools. The questionnaires were reviewed by clinical experts to ensure content validity, but no formal reliability or validity analysis was conducted (see Appendix I). Immediate and long-lasting coolant effects were assessed with dichotomous ‘Yes’ or ‘No’ questions at Days 0, 7, and 14. At Day 21, a five-point Likert scale was utilized to gauge agreement with statements related to pain relief, cooling sensation, injury recovery, and mobility. An Acceptability Test provided subjective feedback on product experience. Product attributes such as odor, color, and texture were rated using descriptive categories ranging from ‘Very Unpleasant’ to ‘Very Pleasant’. This approach allowed for a comprehensive assessment of both the efficacy and user experience associated with the product.
Intervention
Fisiocrem Spray (Uriach, Barcelona, Spain) is a cryotherapy-based spray containing butane, propane, isobutane, and ethyl alcohol as propellants, which ensure the rapid evaporation necessary to produce a cooling effect upon application.16 The formulation also includes natural aromatic compounds such as arnica, St. John’s Wort, calendula, Melaleuca (tea tree oil) and menthol which contribute to the cosmetic attributes of the product, providing fragrance and sensory characteristics.
Detailed application instructions were provided both verbally and in written form, specifying that the product should be self-applied by the participants twice daily, approximately 12 hours apart, directly to the affected area during the initial two weeks (Figure 1). The treatment area corresponded to the size of the injured joint (knee or ankle). Specifically, the spray was applied over an area of approximately 10-15 cm in diameter. The application was performed without soft tissue massage, allowing the product to act topically post-application. The third week served as a washout period during which no product was applied.
Statistical Methods
A descriptive statistical approach was adopted for quantitative biometric variables measured at different experimental time points. Mixed-effects models and multivariate linear mixed-effects models were deployed to discern differences in pain related to the disease and the effectiveness of the product at each time point compared to a reference time. Furthermore, Hedges’ g was calculated as the measure of effect size for improvements. According to conventional thresholds, a Hedges’ g of 0.2 is considered small, 0.5 moderate, and 0.8 or higher large, indicating the strength of the observed effects.17 ROM measures were analyzed using the non-parametric Friedman test for repeated measures. Although the statistical test is based on medians, means are presented in the figure to better illustrate changes over time. Statistical analyses were conducted using the R packages, version 4.1.3, and SPSS, version 26. The significance level for all statistical tests was set at p<0.05.
RESULTS
The recruitment and progression of participants through the phases of this study are depicted in Figure 2. Initially, 60 participants were screened, all of whom were eligible and proceeded to the treatment phase without any screening failures. Throughout the study, there was high participant retention, with only one discontinuation not related to adverse events. Ultimately, 59 participants successfully completed the study.
The study population included participants with varied ages, weights, and heights, as outlined in Table 1. Participant ages, weights, and heights spanned a wide spectrum, indicating a representative sample across various demographic characteristics. Notably, the study included a predominance of male participants, comprising 61.6% of the total.
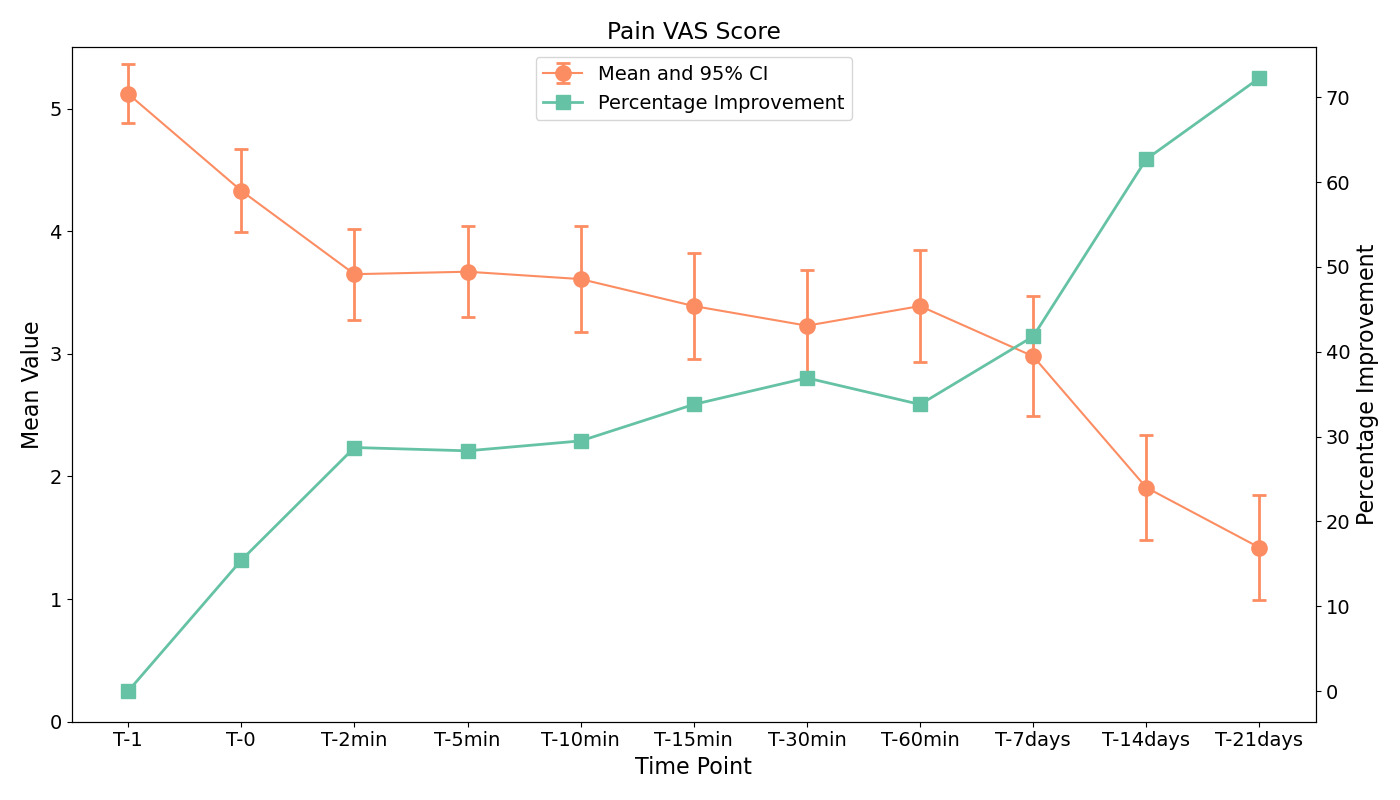
In terms of clinical effectiveness, significant reductions in pain were observed as measured by the Visual Analogue Scale (VAS) from baseline (T0) to each subsequent assessment up to the last day of the trial (T21 days), p < 0.001; Hedges’ g = −1.90 (Figure 3). A reduction of 0.79 at T0 (p < 0.001, Hedges’ g = −0.68) exceeds the Minimal Clinically Important Difference (MCID) for VAS pain scores in patients with musculoskeletal pain, generally considered to be approximately 1.0 cm, indicating a clinically meaningful improvement.18 Similarly, both passive and active joint range of motion category scores showed significant improvements across the experimental timeline compared to the reference time, with median (IQR) scores increasing from 3 (3–4) before spray application to 4 (3–4) at the first two minutes and 4 (4–4) at day 21 (p < 0.001, Friedman test). (Figure 4).
Regarding patient reports of the time to onset of perceived pain relief, at baseline (T0), 73.08% of participants reported relief within 10 seconds. This percentage decreased to 34.55% at T2min but remained relatively stable across subsequent time points (35.19% at T60min and 40% at T14days). The proportion of participants experiencing relief within 1-3 minutes increased from 1.92% at T0 to 36.36% at T2min and remained the most frequent response category at multiple time points. A significant variation in these proportions was observed only at T14days compared to T0 (p = 0.01). The duration of the product’s action showed a slight increasing trend over time. At T0-60min, the mean duration was 2.23 hours (median: 1.9 hours, SD: 2.02). By T7days, the mean duration increased to 3.10 hours (median: 2.1 hours, SD: 2.91, IQR: 4), representing a 39% increase relative to baseline. At T14days, the mean duration further rose to 3.70 hours (median: 2.2 hours, SD: 4.88, IQR: 3), a 66% increase compared to T0-60min. However, statistical analysis using the Wilcoxon Signed Rank test found no significant differences in duration between T7days or T14days compared to baseline (p = 0.055 and p = 0.111, respectively). These findings suggest a potential trend towards prolonged effect, though without statistical significance.
Regarding the perception of the cooling effect, the proportion of patients experiencing this sensation ranged between 93.2% and 98.3% across various time points, with no statistically significant changes observed over the study period.
In terms of product safety, no adverse events were reported throughout the trial. Subjective evaluations revealed that the product’s odor was rated as pleasant or very pleasant by 79% of participants at the initial control (D0), 84% at the 7-day assessment, and 87% at day 14. Similarly, the color of the product received favorable ratings from 64% of participants at D0, 65% at the 7-day control, and 58% at the 14-day control. Product texture was positively evaluated as well, with 78% approving at D0, and 90% giving favorable ratings at both the 7-day and 14-day controls.
In an evaluation of product comfort, 95% of the participants rated the product as either comfortable or very comfortable at the baseline measurement (Day 0 or D0). This proportion remained consistent at the Day 7 follow-up (95%) and notably increased to 99% during the Day 14 assessment. With respect to meeting patient expectations, 86% of the participants felt the product met or exceeded expectations at baseline (D0). This figure saw a significant uptick to 97% at both the Day 7 and Day 14 assessments. In addressing pain reduction effectiveness, 69% of the participants rated the product as satisfactory or highly satisfactory in mitigating pain at the baseline (D0). The approval rate dramatically increased to 94% on Day 7 and further escalated to 96% by Day 14 of the study.
Global efficacy was evaluated by participants’ ratings of the product’s overall effectiveness. The product was rated as effective, highly effective, or completely effective by 75% of the participants at baseline. This rate increased to 92% on Day 7 and 95% on Day 14.
Regarding the speed at which the product achieved its intended effectiveness, 63% of participants found this aspect to be satisfactory or highly satisfactory at baseline. The proportion of satisfied participants rose to 90% on Day 7 and further improved to 94% on
When participants were asked whether the product facilitated ease of administration, 83% either agreed or strongly agreed at baseline. This consensus increased to 93% on Day 7 and 95% on Day 14. Overall satisfaction with the product was deemed satisfactory or highly satisfactory by 73% of the participants at baseline. The rate of satisfaction dramatically improved to 92% on Day 7 and further rose to 95% by Day 14. In terms of immediate cooling effects, an overwhelming 95% of participants affirmed this property of the product at baseline, with a minor increase to 97% at both Day 7 and Day 14 assessments. Regarding the product’s sustained cooling effects, the affirmative responses started at 76% at baseline, rose to 80% on Day 7, and peaked at 92% by Day 14.
DISCUSSION
Although this study is observational, it provides valuable data on the use of the cryotherapy spray. The findings offer insights into efficacy and safety in clinical practice. The study suggests that the intervention may contribute to pain relief and improved joint mobility, though these outcomes could also be influenced by the passage of time and other concurrent activities. The results of this study suggest that the intervention may contribute to pain reduction, as measured by the Visual Analogue Scale (VAS).
From the outset of the treatment at T0, where a moderate effect size (Hedges’ g = -0.68) was observed, to a sustained decrease in pain by T21 (Hedges’ g = -1.90), the data indicate a consistent trend in pain alleviation across all time points (p < 0.001). While the precise mechanisms underlying this effect require further investigation, possible explanations include the transient skin cooling and associated neuromodulatory effects of vapocoolant sprays. Unlike ice-based cryotherapy, vapocoolants primarily act by inducing rapid evaporation-induced cooling, which activates cutaneous thermoreceptors and modulates nociceptive transmission via the gate control theory. This process may lead to a temporary inhibition of pain signaling at the spinal level, thereby reducing the perception of pain. Additionally, the spray formulation contains ethyl alcohol and natural aromatics, which may exert local counterirritant effects, contributing to the observed analgesic benefits. However, as vapocoolants do not penetrate deeply enough to significantly lower intra-articular or muscular temperatures, their efficacy in modulating deep tissue inflammation or edema remains uncertain. The observed improvements in pain and joint mobility could also be influenced by natural recovery processes, continued physical activity, or placebo effects, which should be considered in future controlled studies. The decrease in pain scores over time aligns with the expected natural healing process of acute and chronic musculoskeletal conditions. Without a placebo-controlled comparison, it is challenging to isolate the specific impact of the cryotherapy spray from the body’s inherent healing mechanisms. Future studies incorporating control groups and randomization are necessary to quantify the intervention’s true efficacy.
The improvements observed in both passive and active joint range of motion (ROM), with a notable effect size of 0.77, suggest that the intervention may have influenced musculoskeletal function beyond pain reduction. While the precise mechanisms remain to be fully elucidated, vapocoolant sprays like the one used in this study are known to induce rapid cutaneous cooling, which can modulate nociceptive input by activating thermoreceptors and inhibiting pain transmission via the gate control mechanism. By reducing perceived pain, individuals may experience decreased protective muscle guarding and greater ease in joint mobilization. Additionally, the transient cooling effect may lead to temporary reductions in local muscle tone and reflex activity, facilitating increased ROM. However, unlike traditional ice-based cryotherapy, vapocoolants do not penetrate deeply enough to significantly alter intra-articular temperature or directly reduce inflammation, suggesting that the observed improvements in ROM are more likely related to neuromodulatory rather than direct anti-inflammatory effects. Future research should explore the interplay between cutaneous cooling, neuromuscular inhibition, and functional mobility to better understand the underlying mechanisms.
The importance of the findings is two-fold. Firstly, effective pain management is an urgent clinical need, often affecting patients’ range of motion. Reduced pain and improved range of motion can contribute to better physical functioning, enabling patients to participate more actively in physical therapy or day-to-day activities, positively affecting recovery.
The rapid perception of symptom relief, reported by a significant proportion of participants shortly after application, suggests a potential short-term benefit of the treatment. However, given the subjective nature of pain perception and the influence of factors such as expectancy effects or natural symptom fluctuations, these improvements cannot be solely attributed to the intervention. Nevertheless, if confirmed in controlled settings, such a rapid effect could contribute to increased patient adherence and overall satisfaction with therapy. It is noteworthy, however, that some variation in the time to onset of relief was observed in the two-week control, which warrants further investigation. The study assessed the temporal evolution of the “Duration of product’s action” over distinct intervals. While there was an observed increase in the average duration from 2.23 hours at T0-60min to 3.70 hours at T14days, this was not significantly different.
This observational study lacked a control group, making it difficult to isolate the effects of the cryotherapy spray from natural recovery or placebo effects. Although participants avoided medication and sports, daily activities could have influenced outcomes. Pain relief and improved ROM may be partly due to placebo effects or the natural healing process. Without a placebo-controlled study, attributing improvements solely to the intervention is uncertain.
The three-week follow-up limits conclusions on long-term effects. The sample of amateur football players may not represent other populations, such as older adults or those with severe conditions. Future controlled trials are needed to confirm these findings. While preliminary data suggest potential benefits of the cryotherapy spray, the observational nature and study limitations prevent definitive conclusions.
Future studies could further explore the long-term benefits and potential applications of this treatment in broader clinical contexts, aiming to substantiate its efficacy and practical applicability in routine care settings.
CONCLUSION
The results of this study suggest that the cryotherapy spray may contribute to pain relief and improved joint mobility, though natural recovery and placebo effects cannot be ruled out. The observed reductions in pain and increased ROM align with expected healing processes, and the neuromodulatory effects of vapocoolants may explain the short-term benefits. However, the lack of a control group limits the ability to attribute these improvements solely to the intervention. While the treatment demonstrated high patient satisfaction and a favorable safety profile, long-term efficacy remains uncertain. Future randomized controlled trials are necessary to confirm these findings and determine the broader clinical applicability of this approach.
Disclosures
This study was sponsored by Uriach Consumer Healthcare S.L.U., Barcelona, Spain. The sponsor had no role in the study design, data collection, analysis, or interpretation, nor in the writing of the manuscript or the decision to submit it for publication. The authors declare that they have no conflicts of interest related to this work.