INTRODUCTION
Proper scapular kinematics and effective stabilization are essential components of upper extremity function. The scapula plays a pivotal role in shoulder mechanics and function, contributing to the smooth and coordinated movement of the upper extremity.1 Abnormal scapular kinematics, commonly called scapular dyskinesis,2 and inadequate stabilization have been linked to various injuries, including subacromial impingement syndrome, rotator cuff pathology,3–6 and glenohumeral (GH) instability.7–9 Many systematic reviews identified scapular dyskinesis as a significant risk factor for injury across multiple overhead athlete populations10 including handball,11,12 tennis,13 water polo,14 and other throwing sports.15 These conditions, which can impair function and lead to chronic pain, coupled with a high incidence of scapular dyskinesis in athletic and general populations (81% and 57% respectively)16 highlight the importance of the scapula in overall upper extremity performance. Accurate clinical assessment of scapular motion is crucial for diagnosing dysfunction,17 guiding rehabilitation, and making informed decisions about return to activity, ideally reducing the risk of reinjury and ensuring optimal functional outcomes.
Following injury or surgery, it is critical to assess scapular motion to inform decision-making regarding return to activity or play.18 However, assessing scapular position and motion is challenging due to the anatomical complexity of the scapulothoracic articulation, multiplanar movements, and coverage by soft tissue.19 Accurate measurement of scapular position requires advanced imaging methods, such as computed tomography (CT), magnetic resonance imaging (MRI), or dual fluoroscopy. In research settings, biomechanics laboratories often use electromagnetic sensors or camera-based motion capture systems.20 For the most precise results, sensors or reflective markers are mounted on bone pins placed into the scapula, enabling detailed tracking of scapular movement.21
While precise, these methods are invasive, time-consuming, and costly, limiting their practicality in routine clinical use.22 Therefore, clinicians have historically relied on visual observation to assess scapular motion, albeit with limited reliability and objectivity.23–26 Over time, techniques have been developed to measure scapular motion in static positions, yet these methods still fall short of capturing dynamic, continuous, and functional motions.
Furthermore, although there are numerous studies using simple, objective clinical instruments to measure scapular motion in basic actively achieve shoulder joint positions, there is very little research evaluating scapular dynamics during commonly utilized clinical functional assessments. Such assessments include the Upper Quarter-Y Balance Test (UQ-YBT), Closed Kinetic Chain Upper Extremity Stability Test (CKCUEST), One-Arm Hop Test (OAHT), and Seated Single Arm Shot Put (SSASP),18 and are essential in determining an individual’s readiness to return to high level activity or assessing the risk of future injury. Functional assessments incorporating quantitative measures of dynamic scapular motion would provide more comprehensive information for clinical decision-making, especially in the context of rehabilitation and performance optimization.
The purpose of this scoping review was to systematically identify and summarize the existing literature on clinically feasible quantitative methods that assess active dynamic scapular motion in at least one plane. This review aims to identify and categorize the various assessment tools, technologies, and protocols used in clinical settings to quantify dynamic scapular motion. By synthesizing this information, the review seeks to provide insights into the current state of knowledge, highlight gaps in research, and guide future studies aiming to develop practical and effective tools for evaluating dynamic scapular motion in clinical practice.
METHODS
The final protocol for this scoping review was prospectively registered with the Open Science Framework on May 6, 2024: (https://doi.org/10.17605/OSF.IO/8UY2N). The methods employed were in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR).27
Peer reviewed publications involving primary human subjects research were considered for inclusion. Included articles were published in the English language, reported scapular motion measured in at least one plane with either continuous motion or at least two actively achieved and maintained positions, and used an objective clinically feasible method. Methods deemed clinically feasible were those that a minimally trained medical provider could reasonably replicate in a clinical setting. Data processing was considered clinically feasible if it could be automatically generated by the accompanying software of the instrument or system. The authors considered signal and post-processing to be any method of data manipulation that occurred outside of the hardware/software configuration that was utilized to collect the data. Methods requiring post-processing of raw sensor data through custom programs (e.g., MATLAB, LabVIEW, Visual3D) or advanced outcome measure calculations were considered not clinically feasible. While not all inclusive, a list of clinically feasible methods included: inertial measurement units (IMU), measuring tapes, calipers, manual goniometers, digital inclinometers, and gravity inclinometers. Examples of methods used to measure scapular motion that would not be considered clinically feasible include three-dimensional camera-based motion capture systems, IMU systems without software providing objective measurements of scapular (e.g., Xsens [Xsens Technologies B.V., Netherlands], MoLab [AnyMo, AB Umea, Sweden], InertiaCube [Intersense Inc., Billerica, MA]) or electromagnetic systems such as Flock of Birds (Ascension Technology Corp, Burlington, VT, USA), Polhemus (SPACE FASTRAK, Colchester, VT, USA), and Vicon (Vicon Motion Systems Ltd., Oxford, UK). The following study designs were eligible for inclusion: randomized controlled trials, quasi-experimental studies, cohort studies, case control studies, case series, and case reports. All other study designs were excluded.
A systematic search was conducted using the PubMed, CINAHL, and SPORTDiscus databases from inception through May 2024. The final search strategy entered in the PubMed database was: scapul* motion AND (measurement OR analysis OR assessment) NOT (cadaver OR corpse OR cadaveric OR specimen) NOT animal NOT (MRI OR CT OR Xray OR Fluoro*) AND (english[Filter]). The analogous search strategy entered into the CINAHL and SPORTDiscus databases was: scapul* motion AND ( measurement OR analysis OR assessment ) NOT ( cadaver or corpse or cadaveric OR specimen ) NOT animal NOT ( mri or magnetic resonance imaging ) NOT ( ct scan or computed tomography or cat scan ) NOT ( xray or x-ray or xrays or x-rays or radiographic imaging ) NOT ( fluoroscopy or fluoroscopic ).
All four authors participated in the evidence screening and data charting processes. To facilitate these processes, the team used Covidence systematic review software (Veritas Health Innovation, Melbourne, Australia), available at www.covidence.org, Covidence is a web-based collaboration software platform that streamlines the production of systematic and other literature reviews. To refine and validate the screening criteria and variables for data charting, the authors performed a pilot review of twenty candidate articles. Upon identification and removal of duplicate articles, a review of titles and abstracts was completed in duplicate for all remaining articles. Reviewers voted yes, no, or maybe for each article where a maybe vote was considered equivalent to a yes vote. Full text reviews were then completed in duplicate for all articles that received votes for continued inclusion. The reasons used for exclusion at the full text review stage were non-English language manuscripts, wrong study design, abstract only, unable to obtain full text, wrong outcome, wrong type of range of motion (ROM) outcome, wrong type of instrumentation, and wrong population. Extensive efforts were made to obtain full-text manuscripts by searching standard indexed databases, utilizing academic institution and Federal Government subscriptions, and submitting requests through interlibrary loan services. However, these efforts did not include direct contact with authors. All discrepancies were resolved through majority group consensus.
The data charting tool was developed by all authors and calibrated through pilot testing. The Covidence Extraction 2 template was used to create the data charting tool and to subsequently assess the level of agreement between reviewers. Group consensus was required for a variable to be included in the charting tool. A pilot test of the charting tool was conducted by two authors (WP and MT) and consisted of five randomly selected included studies. The authors observed 100% raw agreement for all extracted variables during the pilot test. All included full text articles were subjected to a duplicate data charting process, and discrepancies were resolved through majority group consensus. The variables collected were title, aim/hypothesis, study design, participant demographics, instrument used to measure dynamic scapular motion, brief description of measurement methodology, number and description of dynamic motions performed, scapular motion measured, outcome units, and key results.
All variables collected as part of the data charting process were imported into a spreadsheet and reviewed for the optimal method of reporting. Many of the variables are reported in narrative format by frequency counts, percentages, and descriptions of observed trends and are presented in table format with studies listed alphabetically (Appendix 1). Methodological studies focused on establishing reliability and/or validity are summarized in table format. The methods used to measure scapular motion, and the summary of scapular measurements are reported using pie charts and bar graphs.
For studies reporting reliability statistics, we considered intraclass correlation coefficient (ICC) values greater than .90 excellent, values greater than .75 good, and values between .50 and .75 moderate. ICC values below .50 were considered poor.28 For studies reporting validity statistics, we summarized the coefficient of determination (Pearson r2) or a Pearson product moment correlation coefficient (Pearson r) for each correlation of two devices where larger values indicated a stronger relationship. Pearson r values between .25 and .50 were considered low to fair, between .50 and .75 moderate to good, and greater than .75 strong.28 Instruments used as reference standards for validating clinically feasible measures may include previously described non-clinically feasible tools, such as three-dimensional motion capture systems or electromagnetic systems, which are commonly used to establish the validity of more practical instruments. Additionally, evaluating the reliability and validity of various instruments was not the focus of this review; therefore, data from studies reporting reliability and validity were included even if the outcomes and statistics required additional calculations and data processing.
RESULTS
The systematic search identified 2,689 studies across the PubMed, SPORTDiscus, and CINAHL databases. After removing 440 duplicates, 2,249 unique studies were screened. Following a review of titles and abstracts, 2,010 studies were excluded. The level of raw agreement between reviewers at this stage was 96.8%, and Kappa was .84 (.80, .89). Most of these exclusions were due to the use of clinically unfeasible instrumentation, such as electromagnetic tracking systems or marker-based motion capture systems, the absence of dynamically achieved positions, studies focusing solely on GH motion, or being review articles. Additionally, six studies were excluded because the full text was not available.
After the title and abstract review, 233 studies underwent full-text evaluation. Of these, 171 were excluded based on inclusion and exclusion criteria. The level of raw agreement at this stage was 92.9%, and Kappa was .84 (.71, .97). The most common reasons for exclusion at this stage included the use of inappropriate instrumentation (123 studies) and the assessment of incorrect range of motion outcomes that focused solely on GH motion (38 studies). Ultimately, 62 studies4,29–98 met all inclusion criteria and were included in the final review (Figure 1).
The 62 studies involved a total of 3,210 participants, with individual study sample sizes ranging from a minimum of four participants to a maximum of 364. Participant demographics can be found in Table 1. Healthy participants were featured in the majority of studies, with 72.5% including at least one group of healthy individuals,4,30–36,39–41,44–46,49,51–60,62–66,68–74,76–79,81–94,96 and 19.3% of the studies exclusively involving healthy non-athlete participants.31,33,36,39,41,44,45,63,70,71,73,74,76–79,81,83,93,96 Injured participants were included in 38.7% of the studies,4,29,32,37,38,42,43,47–53,55,61,62,64,67,69,72,80,90,92,95,97 and athletes were represented in 41.9%.34,35,40,42,46,54,56–60,65,66,68,75,82,86–89,91,94,98
Among the included studies, a variety of clinically feasible instruments were used to assess dynamic scapular motion. Digital inclinometers were the most commonly utilized, appearing in 49.3% of studies (Figure 2) while bubble or gravity inclinometers were used in 21.7% of studies. Other instruments included measuring tapes (used in 7.2% of studies), universal goniometers (used in 5.8% of studies), and IMUs (used in 2.9% of studies).
The number of dynamic motions assessed varied across the studies. In 19.3% of studies, only two dynamic motions were assessed, while 37.1% of studies evaluated three dynamic motions. Four dynamic motions were assessed in 25.8% of the studies, and continuous motion was analyzed in 6.5% of the studies. Regarding the types of dynamic motions performed, frontal plane GH abduction was the most. GH scaption was the next most commonly evaluated motion, performed in 38.7% of studies.
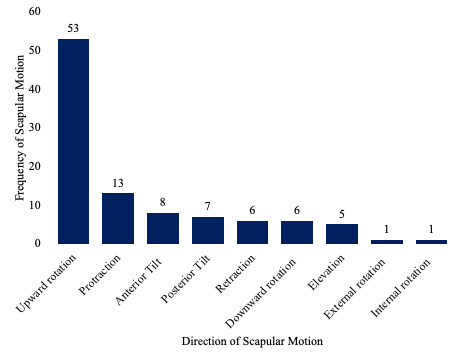
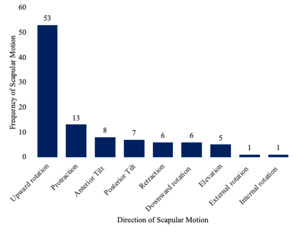
The assessment of scapular motions predominantly focused on upward rotation (UR), which was measured in 85.4% of the included studies (Figure 3). Scapular protraction was the second most evaluated motion at 20.9% of studies, followed by anterior tilt which was measured in 12.9%. The outcomes used to quantify dynamic scapular motion were primarily reported in degrees, with 91.9% of studies utilizing this metric, while distance was reported in 16.1% of studies.
Table 2 summarizes the extracted data that establishes the degree of reliability and validity associated with the various scapular motion measurement methods. Specifically, 9.6% of included studies reported criterion validity results when comparing a clinically feasible method with an established reference standard. In general, the reported level of predictive accuracy between the clinically feasible devices and their respective reference standards was high. Digital inclinometers that assessed scapular motion within the GH scaption plane were studied most frequently and their associated range of Pearson r values suggest a moderate to strong relationship with their respective reference standards.
Additionally, 19.3% of included studies reported relative or absolute measures of rater and/or test-retest reliability. Across all studies, variability in reported reliability results was observed, with values ranging from poor to excellent. However, the majority of results were less than excellent. Protocols that employed digital inclinometers and measured scapular UR and anterior-to-posterior (A-P) tilt demonstrated moderate to excellent rater and test-retest reliability. An initial study by Watson et al.97 also reported good to excellent intra-rater reliability when measuring scapular UR using two gravity inclinometers. However, subsequent studies using a similar protocol both reported poor inter-rater reliability when measuring scapular UR.39,55 The use of a manual plastic goniometer to measure scapular protraction and retraction also resulted in poor to moderate rater reliability.79
DISCUSSION
This scoping review aimed to summarize clinically feasible methods for assessing dynamic scapular motion and to highlight gaps in the current body of research. The results offer valuable insights into available tools and methods for assessing scapular motion in clinical settings. However, several limitations in the reliability, validity, and practical application of these methods must be addressed to improve the assessment of scapular motion, especially during functional activities.
The findings demonstrate that while a variety of clinically feasible tools, such as digital inclinometers, gravity inclinometers, and IMUs, are available for measuring scapular motion, their reliability and validity are inconsistent when applied to dynamic motions. For instance, while digital inclinometers show moderate to strong validity compared to reference standards,52,76 others demonstrate limited reliability, particularly during dynamic motions. Gravity inclinometers, for example, exhibited excellent intra-rater reliability in static positions,97 but inter-rater reliability was notably poor.39,55 This variability is attributed to the complexity of scapular motion, subjectivity inherent in using manual tools, and inconsistent assessment protocols. These findings align with D’Hondt and colleagues’ systematic review, which concluded that insufficient evidence existed to recommend any instrument for clinical examination.99 A key difference between their review and the current review is that theirs was limited to original validation studies and did not require instruments to be tested in multiple dynamic motions. Additionally, validity was assessed in only seven studies, of which four were compared to electromagnetic sensory systems,52,53,76,77 one to marker-based motion capture,33 and two to other manual instruments.49,93 None established validity against the current gold standard of bone-pin mounted sensors.
Another limitation of the clinically feasible instruments identified in this review is their predominant focus on UR of the scapula. UR was measured in 85.4% of the studies and was the sole motion assessed in over half (40 studies). Other important motions, such as A-P tilt and protraction/retraction, were underreported. This is concerning, given that posterior tilt of 44° and external rotation of 27° are required for full glenohumeral abduction,21 not to mention the multiplanar motions necessary for various functional activities.
Clinicians require tools that can assess continuous scapular motion during functional activities, including reaching, throwing, or sport-specific movements. Scapular dyskinesis often only becomes evident during dynamic tasks, making methods that capture motion across multiple planes essential for accurately diagnosing dysfunction and guiding rehabilitation. It is notable, then, that only one study used a functional task, performing virtual rehab in a simulated kitchen.33 Furthermore, only 6.5% of studies evaluated continuous motion, two employing IMUs67,73 and two using less reliable and valid tools such as the Microsoft Kinect33 and an iPad.92 Although several studies incorporated IMUs during continuous motion assessments36,37,41,45,66,69–72 including one study focused on functional tasks like combing hair, fastening a seatbelt, and placing a cup on a shelf,37 these were excluded due to their lack of clinically feasible, automated measures for capturing scapula-specific motion.
The clinical relevance of scapular motion is most apparent during dynamic and functional tasks. Static assessments fail to capture the complex interactions between the scapula, humerus, and thoracic spine during activities requiring coordinated motion across multiple joints. This lack of functional assessment poses a challenge for clinicians when evaluating an individual’s readiness to return to high-level activity, particularly in athletes or physically active individuals. Notably, no studies provided clinical, objective measures of scapular motion during standard functional performance assessments,100 such as the Closed Kinetic Chain Upper Extremity Stability Test, the Seated Single-Arm Shot Put Test, pull-ups, or the Y-Balance Upper Quarter Test. Without objective assessments, clinicians rely on basic performance measures like repetitions or distances, which do not provide insight into the quality of motion or the patient’s ability to effectively stabilize the scapula.
This review highlights the scarcity of objective clinical assessments of dynamic scapular motion in both simple and functional shoulder girdle motions. While there is a large body of research on scapular kinematics, unfortunately a large majority of studies employ laboratory-based instrumentation, as evidenced by the 123 studies that were excluded during full-text review. Furthermore, frequently used traditional assessments of scapular dyskinesis, such as the Lateral Scapular Slide Test and the Scapular Dyskinesia Test, are limited as they are subject to observer bias due to their reliance on subjective visual grading.17 Moreover, these tests typically focus on static positions or simple motions, failing to provide a comprehensive evaluation of scapular movement during more complex or sport-specific tasks.
As awareness of the importance of scapular mobility and stability in upper extremity function grows, so does the need for objective and reproducible methods of assessing dynamic scapular motion. Tools that can reliably quantify movement during functional activities and standard performance assessments could improve diagnostic accuracy, guide rehabilitation, and enhance return-to-activity decision-making.
There is a clear need to develop clinically feasible tools that offer both reliability and validity for more comprehensive dynamic scapular assessments. Instruments that can evaluate scapular motion during functional activities, such as throwing or other sport-specific movements, would significantly benefit clinical practice. Clinicians must be able to objectively assess scapular motion across multiple dynamically achieved positions to improve injury prevention, rehabilitation, and performance optimization. Future research should focus on refining existing tools or developing new technologies that meet these criteria. Wearable sensor technology, such as IMUs, offers significant potential due to its portability, affordability, and capacity to capture motion across multiple planes. However, further research is needed to develop software capable of generating automated clinical measures of scapular motion, while validating these methods against established criteria, enhancing reliability, and integrating them into functional movement assessments.
This scoping review has limitations. Primarily, given the variability of study designs, and inherent to scoping review conventions, the authors did not appraise the quality of the included studies. This was a deliberate decision, as the review’s goal was not to assess study quality as would be required for a systematic review or meta-analysis, but rather to report on the instruments used and their relative frequencies. Another potential limitation is the authors’ operational definitions of “dynamic motion” and “clinical feasibility.” Dynamic motion was defined as involving continuous motion or at least two actively achieved positions to distinguish from static measurements that were taken at rest or end range, aiming to more closely relate to functional activities. Additionally, while clinicians can acquire skills in advanced data post-processing, such procedures are not common or feasible in most cost and time-constrained clinical environments.
CONCLUSION
This scoping review identified a variety of clinically feasible methods for assessing dynamic scapular motion but revealed significant gaps in the reliability, validity, and functional application of these tools. Current methods are limited in their ability to assess dynamic scapular motion, particularly during functional activities. Advancing clinical practice requires the development of valid, reliable, and clinically feasible instruments that can assess scapular motion during tasks relevant to daily life and sport. Addressing these gaps will improve the diagnosis of scapular dyskinesis, guide rehabilitation, and enhance outcomes for patients with upper extremity injuries.
_flow_diagram_f.png)

_flow_diagram_f.png)