INTRODUCTION
A century ago, muscle scientists began a quest to understand the “force generation” capability of muscles – by measuring the force levels attained and heat produced when isolated muscle fibers are stimulated to contract. The attention then was not on the fact that it takes skeletal muscle groups in vivo more than a second to attain those final force levels; whereas in many dynamic tasks, there is often only a fraction of a second available to develop and apply the submaximal forces that are needed against encountered loads. To give just one example, simple ambulation does not require high forces; but leg and ankle muscles must quickly develop the relatively small forces that are needed to allow one to confidently move some of their body weight off balance forward, then quickly/briefly re-support it, and keep it moving forward.
Some tasks require high forces. However, the rate at which muscle groups can develop/apply timely submaximal forces to skeletal segments in order to prevent, cause, or add to motions of those segments, also deserves attention. This capability becomes especially pertinent in athletic activities such as running, jumping, and throwing. A lack of scrutiny of muscle force-development rates against static and dynamic loads, may be why there has been no mention, in muscle physiology literature, of the physics concept that mechanical energy must first be generated at adequate rates within muscles’ contractile cells, so external forces are developed within some crucial time interval.
CONTEMPLATING THE ENERGY UNDERLYING SOME NOTABLE FORCE DEVELOPMENTS AGAINST LOADING BODIES
Forces can often be felt; however, while the energy driving those force developments is intangible, it is not irrelevant. Thus, astronomers are now looking for the source of the “dark energy” that apparently is causing the universe to expand at an unexpected rate. Fortunately, to facilitate human mobility, at least over the last century, engineers learned that by controlling the rate that hydrocarbon molecules are combusted – and converted into mechanical energy – useful forces are developed against loads that can move cars, trains and planes. However, a recent review paper1 indicated that a consequential effect of the rate that muscles are able or allowed to convert chemical energy into mechanical energy – enabling requisite, timely forces to be developed when loads are encountered – was seemingly not considered by those authors. Basic physics tells us that any-sized moving bodies, e.g. flowing rivers, rolling bowling balls, swinging baseball bats, etc., all possess mechanical energy; however, that energy does not cause any new forces to develop unless/until it encounters loading bodies – e.g. dams, baseballs, bowling pins, etc. The rate that mechanical energy is supplied and is partly transferred to yielding loads, must also be considered. Has an inadequate consideration of applicable mechanical energy and load dynamics hindered full recognitions of how muscles contract and are neurally controlled? Did neuromuscular scientists overlook evidence, and a pertinent reason, besides risky force levels, why a neural mechanism apparently directly and significantly restricts the contractile power-rates of entire muscle groups if/when one attempts to move/accelerate typical weight loads maximally – to avoid possible end-of-range injuries to joints, or other body parts?
EARLY PHYSICAL EVIDENCE OF LOADING-SPECIFIC NEURAL RESTRICTIONS OF SKELETAL MUSCLE CONTRACTIONS
An indication of neural restrictions on the intensity of muscle contractions under some types of loading was suggested by the unusual results of a 19782 study of skeletal muscles’ force-velocity and power-velocity relationships – not under conventional weight loading – but under isokinetic loading (provided by a muscle dynamometer). After that evidence was reviewed at the 1984 McMaster International Symposium on Human Muscle Power,3 a few of the eminent neuromuscular science attendees unexpectedly (and ironically} questioned the validity of those results – because they differed with the results and conclusions of an earlier 1950 study4 of skeletal muscles’ force-velocity relationships when weight loads accelerate, by D.R. Wilkie, who was one of the invited attendees, and had just given the keynote address.
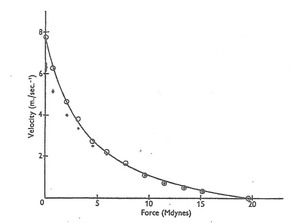
To review, Wilkie found that when his subjects used their elbow-flexing muscles to strenuously accelerate various sizes of free weights, the calculated average, acceleration-driving force levels developed not only varied with loading velocities, but in a hyperbola-curved relationship that looked similar to that exhibited by the neurally isolated, maximally stimulated muscle strips first tested by A.V. Hill in 1938,5 and retested in 1970.6 (Figure 1)
Wilkie stated that his data showed that “the degree of excitation is constant,” and not “a property of the central nervous system.” Wilkie’s force-velocity study usefully showed that when in vivo muscles are recruited to attain their highest permissible force levels against various sizes of free, and thus accelerating weights, those force levels immediately declined from isometric levels – with lower weights and higher loading velocities – like neurally isolated and maximally excited muscle preparations. However, as will be revisited herein, later evidence indicated that while Wilkie’s muscles did exhibit a nearly constant power-rate – reflecting a uniform excitation level – it was likely not maximal, and the average force levels attained by his subjects’ muscles were not what would be expected if there had been no neural limits on the actual maximum possible degree of excitation that Hill’s isolated, purposely maximally stimulated muscle strips surely reached and exhibited.
The 1978 study2 showed that under isokinetic loading, which allows muscles to freely reach a selected loading velocity but thereafter prevents any further acceleration above that loading velocity, muscles are allowed to attain virtually the same force levels as they can in isometric contractions, even up to selected low/midrange, isokinetic loading velocities. (Figure 2)
Note, in that study, the force levels that knee-extensor muscles attained with very intensive efforts at various controlled velocities, at 30 degrees before full extension, were assessed. This was a functional knee position, and allowed the muscles enough time to attain/display their full force potentials at the higher velocities. At the lower velocities, the very intense efforts were started midway through the range of motion, so they did not have to be maintained until they had (noticeably) quickly started to fade. If appropriate contraction times are not provided for in force-velocity studies (as Hill did), the results cannot be compared with Hill’s findings to discern the effects of any neural influences. The 1978 study2 indicated that even under isokinetic loading, a neural mechanism apparently keeps the highest attainable force levels of in vivo muscle groups at low loading velocities well below (as much as 50% below) the actual maximum, low-velocity force potentials of isolated, maximally stimulated muscles.
Wilkie did not calculate the externally manifested, underlying power-rates his test muscles had operated at; however, one can see from the hyperbolic relationship he graphed (Figure 1), that the product of force times velocity was nearly uniform (see following note). This could also have meant that in all his weight acceleration tests, those muscles’ contractile power-rates were also limited to a submaximal level by some neural mechanism. In the 1978 study of the force-velocity relationship of muscles under isokinetic loading,2 instantaneous muscle power-rates first rose markedly (Figure 3) as higher loading velocities were set, until the muscles appeared to finally reach the intrinsic contractile power-rate limit that Hill’s maximally stimulated muscle strips exhibited. Thus, it appears that because of the apparent neural restriction on high force levels visible in Figure 2, the highest power-rates attainable by healthy knee-extensors – under isokinetic loading – did not occur until the force attained at a selected loading speed of 240 degrees per second or higher was tested, and quantified by multiplying force and velocity values to obtain the product, which is instantaneous power (as in the formula: P = F x V, where P is power-rate, F is force, and V is velocity). Note, in physics, the term “power” is often used to denote the rate that mechanical energy is generated or is transferred.
THE CONTRACTILE POWER-RATES OF SKELETAL MUSCLES: WHY MIGHT THEY BE QUICKLY, NEURALLY RESTRICTED UNDER FORMS OF LOADING THAT ALLOW FOR ACCELERATIONS?
What determines the “contractile power-rates” of skeletal muscles, and why would they be limited to submaximal levels during contractions against accelerating loads, but not under isokinetic loading? A full answer to this question starts with the rate at which the ATP molecules within muscles’ tiny sarcomere contractile cells are lysed – and their latent chemical energy is first converted into thermal energy, and mechanical energy – likely in series of tiny impulses of kinetic mechanical energy. This energy enables the sarcomeres’ tiny myofilaments to move against viscoelastic resistance and progressively overlap. After Hislop and Perrine described the clinical potential of using isokinetic loading to specifically improve a muscle’s power capability in 1967,7 the mechanical energy basis of that capability was postulated by Perrine in a 1968 article.8 Unfortunately, improved microscopy still has not allowed researchers to clearly observe this dynamic process in real time. However, it can be safely assumed that kinetic mechanical energy is first generated, and is briefly stored, consolidated, and transferred – as kinetic and/or potential energy – via tendons to skeletal segments – which then powers force development at various rates as the merged energy interacts with yielding or unyielding loading bodies.
In dynamic muscle contractions, particularly in athletic actions, the rate at which force develops or persists, often depends on the rate that mechanical energy is supplied, versus the rate it is being transferred to an accelerating loading body – as in Newton’s formula F = ma, where F is force, m is mass, and a is acceleration. By 2015, because neuromuscular research literature still had shown little interest in quantifying either the core/contractile, or externally (FxV) manifested, composite contractile power-rates of muscle groups, and continued to be focused on their easier to measure, time and position-disregarded, peak-attained, force levels, some reasons to consider those core energy-conversion power-rates were offered by Perrine in a 2015 article.9 That article included a first-time extrapolated, side-by-side display of three force-velocity relationships of isolated animal skeletal muscles, and in vivo human skeletal muscle groups contracting under different forms of loading. (Figure 4)
This comparison indicates that two separate neural mechanisms may restrict the contractile intensities of muscle group contractions – not just to avoid risky, attained force levels, but also to vigorously guard against time-related injury risks created by load accelerations. Thus, a more modern way was suggested to directly measure the force levels attained by in vivo muscle groups contracting against accelerating weights in order to assess Wilkie’s experimental data,4 not his stated conclusion that indicated the absence of any neural restriction.
The author of this commentary has suspected for sixty years that our impressive muscle-recruiting, yet self-protective neuromuscular systems have functional intelligence, and “understand” the relationships between mechanical energy generation rates, and dynamic force developments, under different loading conditions. If so, it would make sense they would have injury-risk-avoidance mechanisms for handling the temporal risks of injury from contractions made under some loading conditions, but not all loading conditions.
To support that perspective in the earlier cited 2015 article9 by this author, it was proposed that when agonist muscles are contracted maximally against sub-maximal weight loads, in addition to any risky force levels attained, presumably detected by Golgi tendon organs, the acceleration of those loads may also present impending, end-of-range joint or other body injury risks – which muscle spindles in antagonist muscles seem aptly positioned to detect, and perhaps via interneuron connections in the spine, lessen these risks, by preemptively inhibiting (by modulating firing frequencies, and/or the number and synchrony of motor units) the agonist muscle contractions that are powering them. Note: neuroscientists have known that spindles in agonist muscles act – via interneuron connections in the spine – to reciprocally inhibit opposite antagonist muscle contractions whenever agonist muscles contract.10 The CNS researchers whose work was surveyed in the previously noted 2017 review paper1 were perhaps not aware of the 2015 physical evidence9 that another trigger and mode of reciprocal inhibition may also occur. Could it be that the CNS regards the future injury risk posed by accelerating limbs/body segments as more problematic than just the immediate velocity that some antagonist muscle is stretching or lengthening? Although Wilkie did not recognize it, his results4 may have indicated that some part of the CNS quickly restricted the contractile intensities/power-rates of his subjects’ elbow flexing muscles when his test weight loads were accelerating.
ASSESSING AND IMPROVING MUSCLES’ POWER-RATE CAPABILITIES
In 1960, James Perrine, a bioengineer/inventor, conceived of isokinetic loading after noticing that it was perceptively harder to make or sustain a high-effort contraction against an accelerating load, than against a nonaccelerating load. Typically, when scientists develop a theory, they conduct a study to test that theory. However, it took several years to first locate and convince a company (Technicon, White Plains, NY which made blood-assessment instruments) of the broad need for, and value of a speed-controlling dynamometer to assess and train muscles’ dynamic force and power capabilities. It took until 1970 to convince a second manufacturer (Lumex, Bay Shore, NY) to make expensive improvements to the original “Cybex” isokinetic-loading dynamometer/exerciser to extend its test velocity range, so it could determine more of muscles’ force and power-velocity range (even though early purchasers were content that it enabled them to better test and restore muscles’ low-speed maximal force “strength” capability). Perrine then designed the “Kinetron” pre-ambulation trainer, the “Fitron” isokinetic-loading, cycle ergometer, and the “Vertec” vertical jump-height tester/trainer – the latter to demonstrate a familiar athletic action that surely depends on muscles’ power-rate capability. It took Perrine several more years to develop a relationship with a neuromuscular research laboratory under the Department of Kinesiology at UCLA, in order to conduct the earlier cited study2 of the force-velocity and power-velocity relationships of skeletal muscles’ when contracting under isokinetic loading.
Are the power-rates of skeletal muscles currently receiving apt scrutiny by PTs, Sports PTs, and ATCs? Can those power-rates be usefully assessed with isokinetic-loading dynamometers? It appears they can be2,9,11 as long as one provides for an adequate loading velocity, force-development time, and for the apparent neural restriction of in vivo muscles’ force levels at low/medium loading velocities. As noted herein, those higher force levels are evidently neurally restricted under both isokinetic loading and free weight loading. In addition, unlike low-speed isokinetic tests wherein muscles have enough time to attain their highest allowed force levels, which can readily be felt, in higher-speed tests, it can be very challenging to contract muscle groups intensively enough so they can briefly attain their actual highest contractile power-rate capability – without visual feedback. A recent U-tube video (https://youtu.be/0gSWLJ9tb3c) shows a motivated athlete attaining a remarkable 50-inch vertical jump height on a Vertec – which provided immediate feedback on the power-rates he presumably attained by several consecutive, power-rate-optimizing muscle contraction efforts.
Isokinetic-loading devices can provide immediate, visual feedback on muscle force levels, including the small force levels that are briefly attained when a sufficiently high loading speed is set. A loading speed of 180 deg/sec might be high enough to reveal the highest power-rate attainable by untrained knee extensor muscles; however the previously discussed 1978 study2 by Perrine & Edgerton, of a mix of athletes and laboratory personnel indicated that a loading speed of at least 240 deg/sec might be better to test the power-rate capabilities of athletes’ knee extensor muscles – to fully avoid the effects of any neural restrictions on their often higher dynamic force levels – that are still at or near the highest allowed levels. The visual force feedback provided by an isokinetic-loading device should be visible to the person whose muscle-power-rate capability is being tested or trained.
Also, to assess the power-rate capabilities of muscle groups, just noting the peak force attained in contractions over a range of movement at high speed under isokinetic loading, is not sufficient – because the muscle-length/joint-position at which that peak force occurs varies with loading speed/time, and may occur before the muscles have had enough time to reach their full force potential, especially at a high loading speed. Thus, a muscle group’s power-rate-dependent, dynamic force capability, exhibited under isokinetic loading, should be quantified at a point later in a joint’s range of movement that is beyond a position where maximum force can be attained and/or restricted. In the previously discussed 1978 study2 of the force and power-velocity relationships of subjects’ knee extensor muscles, that point was 30 degrees before full extension.
A recent paper12 by Wilk et al. considered the inability of many athletes to return to their prior levels of proficiency after injuries and/or surgery, particularly after anterior cruciate ligament surgery. The authors suggested that isokinetic testing remains the best single method to objectively determine muscle strength, power, rate of force development, and endurance. The authors also noted that it has been reported that a quadriceps strength value of 90% or better, when compared to the contralateral (uninjured) knee, was one of the four or five criteria now considered to reduce the risk of reinjury following anterior cruciate ligament surgery. Surely, another criterion should also be considered: quadriceps’ measured power-rate capability.
SUMMARY AND CONCLUSIONS
The brief contraction times (a fraction of a second) occurring during many activities, and the final load speeds desired in some athletic activities, make the power-rate capability of skeletal muscles an important consideration in general and/or in sports-medicine rehabilitation. In many clinical cases, it could be a more important consideration than the maximal force/strength capability of a muscle group – that has been tested and relied on for many decades. This author suspects that when the tendons or ligaments of athletes’ joints are surgically repaired, if the power-rate capabilities of associated joint muscles are not substantially regained by suitable training, athletes return to their prior levels of sports proficiency may not be achieved. Additional controlled studies are needed to determine if/when training to specifically remedy muscle power-rate deficiencies – by exercising on isokinetic-loading devices, or by any other means – are more effective than high-load strength training. It is hoped this commentary might advance this needed research by addressing an apparent, lingering, limiting, knowledge gap.
Corresponding Author
James J Perrine
Oro Valley, AZ
Email: jjperrine@icloud.com
Conflicts of Interest
The author reports no conflicts of interest.