INTRODUCTION
Exercise-induced bronchoconstriction (EIB) is characterized by transient narrowing of the airways during or after exercise that commonly occurs in subjects with asthma or in some athletes with ventilatory disorders.1 The prevalence of EIB is higher in elite athletes, especially those who practice endurance sports.2 However, the occurrence of EIB depends on several factors, including allergic predisposition, training environment, and exercise intensity.3 In addition, elite athletes who sustain high ventilation levels (above 150 l/min) for prolonged periods are more vulnerable to the induction of EIB.4 The risk of EIB increases in atopic subjects with asthma; however, no difference in performance has been observed between atopic and non-atopic endurance athletes.4
Elite endurance athletes (EAs) are regularly exposed to excessive aerobic training loads, which improves the cardiorespiratory system and reduces the risk of developing diseases such as cancer, obesity, and diabetes; however, it may be harmful to their health.5 Furthermore, evidence has demonstrated that elite athletes can develop overtraining syndrome, a response to excessive training that can affect different systems, including the immune and respiratory tract, and induce muscle injuries, EIB, and reduced performance caused by excessive training or lack of rest.6
The prevalence of EIB in the general population varies from 5% to 20%, and is higher in elite athletes, with a prevalence between 30% and 70%. In EIB athletes exhibit an abnormal airway response to intense exercise exposure, which decreases their performance.7 Extreme ventilation can cause airway epithelial injuries, releasing inflammatory mediators, such as IFN-γ and IL-4 in the airways, that are associated with EIB in EAs.8 Furthermore, a recent study showed that marathon runners present increased levels of inflammatory mediators, such as IL-8 and IL-10, after competing, which can modulate the allergic response and EIB appearance.9 The Global Initiative for Asthma (GINA)10 recommends using inhaled corticosteroid (ICS) associated with formoterol to reduce EIB for all people with asthma diagnosis symptoms. The World Anti-Doping Agency (WADA) does not consider pharmacological treatment as doping for athletes with asthma or with other ventilatory disorders. However, despite all the changes induced by training in elite athletes, the effect of proper pharmacological treatment to lessen EIB and the allergic inflammatory response (AIR) remains poorly understood. Although previous studies have suggested using long-acting beta-agonists (LABAs)11 or inhaled corticosteroids (ICSs) as treatments for EIB,12 a treatment using both medications has not been utilized in elite EAs. In the present study, The authors hypothesized that pharmacological treatment (ICS+LABA) could reduce EIB levels and change the AIR and performance in athletes after individualized pharmacological treatment. Therefore, the aim was to examine whether pharmacological treatment can reduce airway disorders such as exercise-induced bronchoconstriction (EIB) and allergic inflammatory response (AIR) in EA.
MATERIALS AND METHODS TRIAL
DESIGN & PARTICIPANTS
Elite EAs with ventilatory disorders, but no history of asthma were included in this interventional study. The inclusion criteria were as follows: males between 18 and 40 years old who compete at the elite level according to previously defined criteria.1 Briefly, the performance criteria for elite endurance athletes were the ability to run 10 km in <33 min, a half-marathon in <1 hr and 10 min, a marathon in <2 hr and 30 min, or an Olympic triathlon in <2 hr and 5 min.13 The study was approved by Ethics Committee of the Clinical Hospital of the University of São Paulo (number 32476014.6.0000.0065), which was carried out according to the Declaration of Helsinki.
Study design
The EA were not under current medical treatment and did not exhibit any symptoms, and before the study, there was no medical or clinical record of respiratory disease. They were assessed during two hospital visits before the intervention and 30 days after the intervention for the final assessment. All the athletes underwent lung function and eucapnic voluntary hyperventilation (EVH) testing. The FEV1 was measured immediately after the challenge and at five, 10, 15, and 20 minutes after EVH. Allergic symptoms were assessed (Allergic Questionnaire for Athletes [AQUA®]) at their first visit. On their second visit, they were subjected to cardiopulmonary exercise testing (CPET), and blood samples were collected to assess the AIR (T helper [Th]1, Th2, and Th17 cell culture), inflammatory mediators (interleukin [IL]-6, IL-1b, IL-8, Macrophage inflammatory protein [MIP], Interferon-gamma inducible protein-10 [IP-10], monocyte chemoattractant protein-1 [MCP-1], Regulated upon activation normal T cell expressed and presumably secreted [RANTES]), and blood cortisol and IgE levels. Finally, saliva was collected (for IgA), and breath exhalation was performed to assess airway inflammation (fraction of exhaled nitric oxide [FeNO]). All hospital visits occurred in the morning between 07:00 and 09:00. Athletes presenting a negative response or no airway ventilation changes after to EVH were included in the control group (n = 18) (CON), while those presenting a positive response (drop in forced expiratory volume in the first second [FEV1] >10%) were included in the treatment group (n=13). Due to the type and design of the study and proposed intervention, no athlete was blinded. Those who were monitored received guidance from a specialist sports doctor.
Intervention
The pharmacological treatment was implemented for the first time to EAs and included individual doses of ICSs and LABAs. As the main criterion for inclusion in the EIB+/CON group athletes should present with exercise-induced bronchoconstriction (EIB) after an EVH test, as previously shown.14 Only if necessary, as a secondary criterion, the specific IgE levels higher than 0.35 KUA was considered.13 Also, only if necessary, the third criterion was used by the allergic or respiratory symptoms based on AQUA© questionnaire scores15 although it is subjective and not specific/objective like IgE. Participants who did not meet any of the three criteria for inclusion in the EIB+ group were allocated to the CON group and did not receive any treatment.
Pharmacological treatment
The pharmacological treatment carried out by a respiratory physician and in accordance with the Global Initiative for Asthma (GINA).1 Athletes of the EIB+ group underwent a medical consultation before the beginning of the pharmacological treatment, which lasted 30 days. Briefly, individually, athletes with mild EIB (≥10% and <25% with a slight drop in FEV1) were prescribed ICS (budesonide) daily associated with inhaled long-acting β2-agonist (LABA; formoterol fumarate dihydrate). Those with moderate EIB (≥25% and <50% with a slight drop in FEV1) were prescribed higher doses of ICS and LABA 400-800 micrograms (mcg) and a short-acting β2-agonist 12 mcg per day. Finally, athletes with severe EIB (≥50% with a slight drop in FEV1) also received montelukast daily. EIB classification was performed as previously described16; details are provided in the supplementary material. All medicines were prescribed according to GINA10 and allergic rhinitis and their impact on asthma.17,18 In addition, the doses of every medication were performed according to the WADA guidelines.19 The CON did not receive any medication or intervention and underwent the same initial and final evaluations.
Assessment
Details of lung function, EVH, CPET, allergic history (AQUA) and other evaluations are provided in the Supplemental Digital Content.
Data Analysis
Both the CON and EIB+ groups underwent the same assessments after the intervention and were compared pre- and post-intervention. All data comparisons were performed using SigmaStat 12.1 (Systat Software, San Jose, CA, USA). Data normality was assessed using the Kolmogorov-Smirnov test, and variable homogeneity was assessed using the Levene test. Group comparisons were performed using unpaired t-tests. The Shapiro-Wilk and the Holm-Sidack post hoc tests were performed for parametric variables. The Friedman test was used for non-parametric variables for inter- and intra-group comparisons before and after pharmacological treatment. The association was analyzed using the Spearman correlation test. The effect size was calculated using the Cohen method and classified as small (0.21–0.49), medium (0.50–0.79), or large (≥0.80).20
RESULTS
One hundred and three elite male athletes were invited to participate in the study; 40 athletes were eligible for the study based on their performance, and 31 were included. Nine athletes were excluded, and the main reasons were i) inability to attend reassessments (n=4), ii) lack of time (n=2), iii) injury during the experimental period (n=2), and iv) other reasons (n=1). No difference between CON and EIB+ was observed in anthropometric characteristics, training volume, lung function, allergic symptoms, IgE levels, and systemic and airway inflammation at baseline (Table 1). In addition, 3 (16%) athletes in the CON and 7 (53.8%) in the EIB+ group exhibited allergic symptoms according to the AQUA© (>5 points).
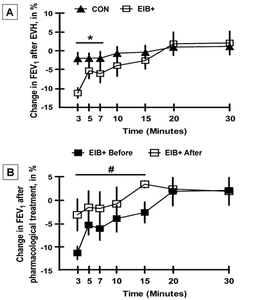
Before the intervention, it was identified that nine athletes reached maximal EIB 3 min. after the EVH test. Additionally, one athlete reached maximal EIB at 5 min. after the EVH test, one at 7 min., one at 15 min., and the other at 30 min after the EVH test. All athletes showed a greater decrease in the FEV1 after exercise (Figure 1).
Figure 1 shows that the EIB+ group exhibited a greater decrease in FEV1 after EVH (p<0.05) than did the CON group (p<0.05). After pharmacological treatment, the FEV1 of the EIB+ group was increased only compared with the baseline at 3 to 15 min after the EVH test (Figure 1, p<0.05). Importantly, the EIB+ group showed an improvement in FEV1 after EVH in the 3rd to 15th minute when compared with the baseline (p<0.05). After pharmacological treatment, only one athlete with EIB+ showed a decrease in FEV1 in the 7th min. after EVH.
Effect of pharmacological treatment on the occurrence of EIB
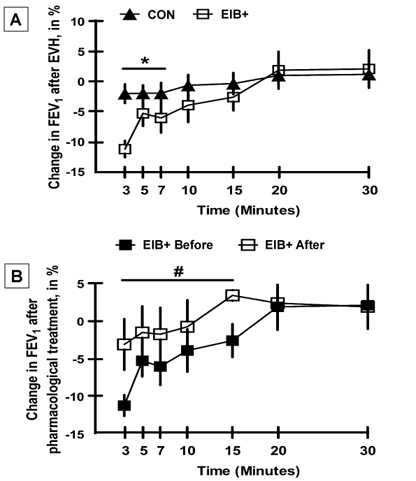
There was no significant difference in FEV1 between the CON and EIB+ groups before and after the intervention (Figure 2; p>0.05). However, the current results showed that 92.3% of the athletes in the EIB+ group did not exhibit EIB (a reduction in FEV1 after EVH) after pharmacological treatment. In addition, only one athlete exhibited a positive response to the EVH test after pharmacological treatment (100% vs. 7.7%; p<0.001 for the proportion of patients before and after treatment, respectively, in the EIB+ group).
Effect of pharmacological treatment on the allergic inflammatory response
There was no difference in the proportion of lymphocytes, naive T cells or T helper subtypes (Th1, Th2, and Th17; Table 2; p>0.05), and cell subtypes between the CON and EIB+ groups before and after the intervention (p>0.05). In addition, no differences were observed in the expression of inflammatory markers IP-10, MIG, IL-6, and IL-1β between the CON and EIB+ (Electronic-Table-1 (E-Table-1) in the Supplemental Digital Content), p>0.05). Similarly, no difference was found in cortisol and salivary IgA levels observed when comparing both groups before and after the intervention (p>0.05). Finally, the MCP-1, RANTES, and IL-8 levels were below the detection limit as showed in the E-Table-1 in the Supplemental Digital Content.
Effects of pharmacological treatment on exercise performance
After the intervention period, the EIB+ group exhibited an increase in speed and peak VO2 during the CPET compared with the baseline and with the CON group (Table 3; p<0.05); however, no difference was observed in the CON group for any moment (p>0.05). There were no differences in the VE, and VE/VCO2 slope, and VE/VO2 in the CON and EIB+ groups between the baseline and postintervention values in any moment (Table 3; p>0.05). The results reported a large effect size comparing the groups before and after intervention in the peak VO2, medium for VO2 at the anaerobic threshold, and small at the aerobic threshold (Electronic-Figure 1 (E-Figure-1) in the Supplemental Digital Content.
DISCUSSION
The results of this study suggeste that endurance athletes (EA) present a reduction in EIB and an improvement in peak exercise performance after 30 days of pharmacological treatment. However, this improvement was not related to any change in the systemic inflammation and humoral and hormonal responses. Therefore, pharmacological treatment can be used to reduce EIB levels without altering the AIR in this population.
Previous studies have shown that EIB reduces the performance of athletes,21–23 and several studies have suggested underlying mechanisms for EIB.22–24 However, guidelines have reported difficulties in treating EIB.14,25 Therefore, studies have attempted to show the importance and effectiveness of pharmacological treatment with an ICS+short-acting β2-agonist (SABA) in athletes from different sports, such as power or mixed endurance/strength training, compared with using only an ICS,25,26 in high-performance endurance athletes at various running distances, such as 10 km to a marathon. The current study produced different results to those from a previous study using fluticasone plus SABA,27 which did not report changes in EIB after EVH, as suggested by the current findings. Other studies have also assessed the effect of SABAs in female cyclists and found an improvement in lung function from baseline, but they did not assess EIB.28 Complementarily, our findings showed that treatment with an ICS (budesonide) and an LABA (formoterol fumarate dihydrate) should be considered when treating athletes with EIB. This treatment contrasts with the previous recommendation of using LABA before exercise and/or leukotriene receptor antagonists or chromones associated. It is important to comment that all β-2 agonists, selective or non-selective, including all optical isomers, are prohibited. However, WADA makes an exception for inhaled formoterol, with a maximum dose of 54 micrograms in 24 hours19; the dose used in our study was 12 to 24 mcg of LABA according to the requirements of each athlete.
Other clinical variables were assessed in addition to EIB, including the AQUA questionnaire, IgE+ levels, and the drop in FEV1. Subsequently, the pharmacological treatment was initiated. Interestingly, the results showed a decrease in EIB (Figure 1), being an important marker of airway changes that become narrowed during EIB,3–7 and a significant improvement in peak VO2 (Table 3; E-Figure-1). The peak VO2 is considered the best indicator of aerobic capacity, and it is used to assess an athlete’s physical capacity and establish intensity training, mainly in elite athletes.29 The current findings suggest that the subjects had high levels of aerobic fitness (Table 3). These results seem relevant because of the increase in peak VO2 of 3.9 (+3.67) ml.kg-1.min-1, representing a 5% increase only 30 days after pharmacological treatment. This increase occurred without any change in the athlete’s training program. Therefore, these improvements led to a large effect size (E-Figure-1) for both the maximal aerobic capacity and increased peak speed. It was also observed that athletes presenting EIB had a higher VE/VO2 in the aerobic threshold and exercise peak, suggesting they required higher ventilation during exercise. These results showed that proper pharmacological treatment could reduce the limitations faced by athletes who present with EIB untreated/undiagnosed. In this sense it has been previously established in patients with asthma that prolonged ICS treatment can not only decrease the severity of EIB but also improve arterial blood oxygenation, which could be a possible mechanism.30
Previous studies have suggested that EIB is associated with changes in the AIR (Th2 response) in elite athletes.9 The AIR was assessed to understand the possible pathways that support the reduction in EIB. The current results suggest that the decrease in EIB was not associated with changes in the AIR or IgE levels. Furthermore, changes in Th1, Th2, or Th17 (Table 2) or antibodies such as IgA,31 were not observed which did not support the hypothesis of the reduction of the EIB by decreasing of the AIR.8 The current findings suggest that pharmacological treatment, particularly corticosteroids, does not reduce the inflammatory allergic response.
Clinical relevance and use of the findings in endurance sports
First, these results reinforce the importance of identifying and adequately treating EIB, aiming to improve the performance of EA. The current results support medication-based interventions to treat EIB in athletes since inhaled corticosteroids and long-acting beta-agonists are not considered doping by the World Athletics and WADA when the presence of EIB is confirmed.19 This study adds new information to the literature, showing that clinical treatment with common and inexpensive medications permitted by the Olympic Committee and WADA can induce benefits for people with high performance in the marathon running.
Athletes, coaches, and exercise physiologists have difficulty identifying EIB. This clinical condition can reduce the athletes’ performance and significantly interfere with the results. The EIB has deleterious effects on the efficiency of gas exchange, which result in a performance decrease,30 induced an impaired in the oxygen uptake in individuals with EIB.32 In this sense, the current findings are also relevant because improvements in performance in elite athletes in a short time (8 weeks) are very difficult to achieve33 and intense exercise training is typically required for at least 8 weeks in these athletes.34 However, these findings suggested a significant improvement after only 30 days. In addition, previous studies have suggested that the assessment of IgE level and allergy symptoms (using AQUA@) can be used to screen for EIB13; however, the current results also reinforce the importance of the EVH test. Finally, the authors observed that athletes who had a lower drop in FEV1 seemed to benefit most from pharmacological treatment. Thus, it was hypothesized that athletes with greater EIB may require either a longer treatment period or an individualized approach.
This study has some limitations. The number of participants receiving pharmacological treatment was small because it was not easy to convince the athletes to participate, this limitation makes it difficult to understand the mechanisms of EIB reduction and to obtain more robust statistics. Therefore, limitations exist in associating the training and competition schedule with study participation, and the study was only possible because the coaches understood its relevance. Interestingly, all those who received treatment reported improvements in their well-being and perceived improvement in their training practices. Importantly, the authors observed that athletes with higher EIB had lower responses in terms of narrowing airways. Another limitation is the lack of a longer follow-up period (3, 6, and 12 months), which can be addressed in future studies. The aerobic fitness was evaluated with CPET in a laboratory which is different of the response of the O2 uptake during a competition performance, despite being considered the gold standard for this purpose. Finally, only a limited number of inflammatory markers were assessed and this could be considered a limitation. Thus, additional studies are necessary, including other inflammatory markers associated with EIB including IL-6, IL-8, calprotectin,35 IL-33-, IL-18-, and IFN-γ.36
CONCLUSION
The results of this study suggest that pharmacological treatment lasting 30 days reduced EIB in elite endurance athletes. However, these benefits were not associated with changes in the AIR. Nonetheless, physicians can consider the use of the pharmacological treatment reported in the present study to treat endurance athletes who present with EIB. Although pharmacological treatment reduced the effect of EIB on athletes, it is possible that with continued use, athletes may develop tolerance to the treatment; therefore, long-term monitoring may be necessary for possible adjustments in medication doses.