INTRODUCTION
Anterior cruciate ligament (ACL) injuries can lead to joint instability, limited range of motion, muscle weakness, and decreased proprioception.1 Indicators for returning to sports after injuries, such as damage to ligament or menisci, include the time that has elapsed since surgery, and assessment of joint stability, range of motion, muscle strength, and performance.2–8 However, some athletes cannot resume their sports even after meeting benchmarks. Researchers have shown that differences in joint movement, muscle strength, and movement patterns can persist for 5–10 years after ACL reconstruction.9,10
Joint motion control relies on feedback from proprioceptors within muscle spindles, Golgi tendon organs, ligaments, and menisci.11 Kennedy et al.12 confirmed the identification of Golgi-like tension receptors near the ACL origin. Additionally, nerve fibers were found in the superficial layer and fibers of the medial collateral ligament, and numerous receptors were identified within the joint capsule, indicating that impaired proprioceptive function in ligaments could lead to instability and laxity of stabilizing tissues, increasing the risk of recurrent injuries. Mullar13 emphasized that ligaments serve as tension receptors, and dynamic stabilizers may fail to function correctly when their length is altered. Zimny et al.14 confirmed three types of receptors in the ACL, underscoring its significance beyond biomechanics and incorporating neurophysiological importance. Additionally, O’Connor et al.15 reported that receptors in animal medial collateral ligaments are closely related to joint angular velocity and reflexes of surrounding muscles.
Additionally, meniscal receptors detect tension during knee movement and transmit this information to the central nervous system, functioning as an early-warning system. Zimny et al.16 confirmed that receptors in the medial meniscus detect compression between the femur and tibia, contributing to proper joint alignment and biomechanical correction. Thus, within the knee, muscle spindles, Golgi tendon organs, ligaments, and menisci contain proprioceptors and are essential for movement execution.
Several researchers have measured proprioception, often by passively moving a joint and having the participant reproduce the position or respond upon reaching the remembered location.1,17,18 Compared with either the uninjured side or healthy individuals, individuals with ACL injuries have reduced joint position sense and kinesthesia.19–22 However, proprioception involves functions beyond recognizing joint position and changes in movements. This implies that assessing only position and motion perception may be inadequate for understanding proprioceptive changes after sports injuries. Proprioception also plays a critical role in sensing muscle and tendon tension levels. As sports movements require precise joint control, athletes should be able to finely adjust muscle output. Consequently, knee joint function should be tested by including tasks that require muscle output adjustments, which is an essential preliminary step for establishing return-to-sport criteria. Moreover, the rapid transmission of proprioceptive information to the central nervous system for efficient control by surrounding muscle groups is crucial. Therefore, peripheral and central nervous functions should be considered.23 The assessment of proprioceptive and central nervous functions, along with mechanical factors and musculoskeletal conditions has been suggested to be essential for determining readiness to return to competition following ligament or meniscal injuries.
Neural function can be assessed using evoked electromyography (EMG),24,25 which involves electrically stimulating peripheral nerves, recording action potentials from the innervated muscles, and capturing reflex EMG waveforms. Despite continued effort, action potentials of a voluntarily contracting muscle undergo a transient suppression following electric stimulation of the mixed nerve innervating that muscle.26 This period of electrical inactivity, designated the mixed nerve silent period, results from several physiologic mechanisms.26 During this silent period, the M-wave, the H-reflex or the F-wave, and the long-latency reflexes (LLR) are recorded. Among these, the H-reflex and F-wave—recorded from the spinal cord to the central nervous system—are used in clinical settings.27–30 In stroke patients, lesions in the sensorimotor cortex or internal capsule can be diagnosed by observing a reduction or disappearance in LLR amplitude on the paralyzed side.28 Abnormal LLR have been observed in various neurological disorders, with reduced LLR amplitude in Huntington’s disease and increased amplitude in Parkinson’s disease.29,31–33 Normal LLR have been observed in Alzheimer’s disease, whereas reduced or absent LLR amplitude has been observed in frontotemporal dementia, suggesting its potential as a low-cost, early biomarker to differentiate between cortical dementia types.34 In ACL injuries, cases where LLR was observed from one to five months postoperatively showed LLR disappearance at six months, when sports resumption became possible.35 Thus, evoked EMG has been utilized for diagnosing neurological disorders and identifying excitability changes in the central nervous system after sports injuries.
In studies by Daikuya et al.6,36 LLR and silent periods of the muscles of thumb opposition were measured during fine adjustments in knee extension force, showing increased central nervous excitability with task difficulty. This indicates that LLR is valuable for evaluating the transmission dynamics from the periphery to the spinal level and central nervous function excitability above the spinal level. Based upon prior research, it was inferred that tasks with higher difficulty levels would require finer adjustments of muscle output. Therefore, this study aimed to examine the relationship between sports injury history or return-to-play status and central nervous system excitability during muscle output adjustments, and to investigate the characteristics of evoked EMG during torque maintenance as a potential indicator for assessing readiness to return to sport. In addition, the hypothesis was that in individuals with previous ligament or meniscal injuries, reduced proprioception may impair the force adjustment capability, which potentially increases the central nervous system excitability during fine force adjustments.
METHODS
Participants: Twenty-one female university basketball players were recruited to participate. Participants were eligible if they had no current musculoskeletal injuries. The study included two groups: (1) Participants with a history of knee ligament or meniscal surgery who had completed a standard rehabilitation37,38 and were cleared to return to play, and (2) Participants with no history of knee injury or surgery. It included both knees of the healthy participants because the knees of the participants with a history of knee injury contained dominance leg and non-dominance leg. To determine the required sample size (number of participants), using Statistical Package for the Social Sciences (version 28, IBM, Armonk, NY, USA), the sample size was estimated using statistical power of 0.8, a standard deviation for each indicator set between 0 and 26, and mean values for each indicator set between 0 and 4. Consequently, a sample size of 6 was calculated for each group.
Procedures: Comparisons between visual and verbal feedback were conducted separately for legs with and without a history of injury. The ethics committee of Hokuriku University approved this study, and all participants were informed about the research and provided written consent to participate (2023-16).
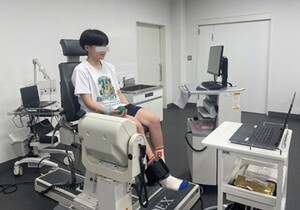
It was measured participants’ maximal voluntary isometric contraction torque in knee extension while seated position with the knee flexed at 60° using the BIODEX system BDX-4X (Biodex Medical Systems Inc., USA) (Figure 1). Participants were provided with a visual target corresponding to 25% of the maximal torque and were instructed to maintain this target torque for 60 s in the same position. During this time, the BIODEX system was synchronized with the UltiumEMG EM-U880 (Noraxon, USA) to record torque data on a personal computer. To assess torque variation during target torque maintenance, we calculated the median frequency of the resulting torque waveform. Data analysis focused on a 10 s segment starting 25 s after the task began. Additionally, during the task, it was recorded LLR data from the ipsilateral upper limb muscle (opponens pollicis) by using the Neuropack MEB-9604 (Nihon Kohden Corporation, Japan). The participants performed a gentle grasping the rubber ball to elicit the contraction of opponens pollicis, because it needed the muscle contraction to evoke the LLR. The active electrode was placed over the muscle belly of the opponens pollicis, with the reference electrode positioned on the dorsal interphalangeal joint of the thumb. It was based the stimulation conditions on the studies by Daikuya et al. (2017, 2018)36,39 as follows: intensity, 120% of the maximal stimulus that induces a maximal M-wave; frequency, 0.5 Hz; duration, 0.2 ms; and number of stimuli, 16 single pulses for each task. It was measured the LLR occurrence rate, latency, duration, maximum amplitude ratio (LLR/Mmax), and phase count. The LLR occurrence rate was defined as the percentage of trials in which LLR was observed. Latency was the time interval from the stimulus onset to the onset of the LLR response. Duration was the time from the onset to the offset of the LLR EMG response. The maximum amplitude ratio represented the peak amplitude of the LLR response normalized to the maximal M-wave amplitude. The phase count referred to the number of distinct EMG bursts observed during the LLR period. It was used the following conditions to attempt to maintain the target torque: visual feedback from the BIODEX (visual condition) and verbal feedback from the examiner (auditory condition) with the participant’s eyes closed.
Statistical methods
The Shapiro–Wilk test was used to confirm the normality of the variables. The Wilcoxon signed-rank test was then performed to compare outcomes between the conditions. The significance level was set at 5%, and IBM SPSS 28.0 for Windows (IBM SPSS Japan, Tokyo, Japan) was used for statistical analysis.
RESULTS
The participants had a mean age of 19.4 ± 1.2 years (range, 18–21 years), height of 164.1 ± 5.5 cm (range, 156.0–174.0 cm), and weight of 61.3 ± 4.3 kg (range, 52.5–68.2 kg) (mean ± standard deviation (range)). Six of the 21 participants had a history of sports-related knee injuries, of which one had not yet returned to sports (Table 1). There were no significant differences in demographic data between participants with and without a history of injury.
Tables 2 and 3 show the median frequency of the torque curve during torque maintenance and various LLR indices, with a representative waveform of the evoked EMG obtained from the injured knee of a single participant shown in Figure 2. Regarding the median frequency of the torque curve during torque maintenance, the visual condition for uninjured knees was significantly higher than the auditory condition (p < 0.05). As for LLR, the amplitude ratio LLR/Mmax in the visual condition for injured knees was significantly higher than that in the auditory condition (p < 0.05). Among athletes with a history of sports injuries, the one athlete who had not returned to competition beyond the scheduled return time exhibited increased the median frequency of the torque curve during torque maintenance and decreased amplitude ratio LLR/Mmax.
DISCUSSION
The effects of different feedback methods were examined on the median frequency of torque curves and various LLR indicators, including occurrence frequency, latency, duration, amplitude ratio LLR/Mmax, and phase count during a torque maintenance task (visual and verbal). The median frequency of the torque curve served as an indicator of torque adjustment ability (i.e., task performance proficiency), while the LLR indicators provided insights into central nervous system excitability beyond the spinal level.
Generally, auditory information is processed faster than visual information; however, visual information tends to provide more useful data40 for balance and postural control.41 The results of several studies indicate that body sway increases when visual input is interrupted during upright posture or walking, emphasizing the significant influence of visual information on postural stability.40,42 Based on these reports, an environment where visual information is available allows for greater physical performance than one relying solely on auditory information. Therefore, it was hypothesized that tasks performed under visual conditions would be less challenging, whereas those performed under auditory conditions would be more challenging.
The median frequency of the torque curve during torque maintenance is an indicator to assess the degree of torque fluctuation while maintaining the target torque and to determine the nature of fine adjustments during the task. A higher median frequency indicates more precise attempts at muscle output adjustments. Previous research has used deviation areas from the target torque line and the number of intersections as indicators, which determined that greater deviation areas indicate larger errors, and a higher number of intersections indicate more frequent attempts at muscle output adjustment.43 These studies reported that fine adjustment methods differ between the dominant and nondominant legs, with the dominant leg showing increased deviation areas and decreased intersection numbers in higher-difficulty tasks.43 It was observed that the mid-frequency of the torque curve during torque maintenance for the non-injured leg was higher under the visual condition (assumed to be of lower difficulty) compared with the auditory condition, indicating that finer output adjustments could be achieved under visual conditions than auditory conditions. Although the specific indicators differ between previous studies and this study, the current findings support previous research, indicating that subtle movements may occur to achieve the intended goal in an environment with available visual information.
LLR recorded from the upper limbs is an autonomic reflex with a long latency obtained from the innervated muscles upon peripheral nerve stimulation, which can be interpreted as being mediated by higher central nervous system pathways beyond the spinal cord.44 LLR is a useful indicator to evaluate conduction dynamics from the periphery to levels above the spinal cord and assess central nervous function excitability above the spinal cord, regardless of disease.28,33,34 Consequently, LLR has been clinically applied, particularly in cases of neuromuscular diseases.29 Key indicators in LLR research include occurrence frequency, latency, duration, amplitude ratio (LLR/Max), and the number of phases. Frequency represents the proportion of waveform appearances relative to the number of stimuli, indicating changes in the excitability in the responsible centers. Latency, defined as the interval from the start of electrical stimulation to the onset of response, reflects the conduction state from the stimulation site to the LLR-responsible center. Duration, which measures the time from waveform onset to its return to baseline, indicates variations due to central nervous function excitability and localization. LLR amplitude and amplitude ratio (LLR/Mmax) serve as indicators of excitability changes in the responsible center and relative central nervous function excitability with the periphery (recording muscle), respectively. Additionally, the number of phases refers to the LLR phase count, indicating the level of excitability in the responsible center. These indicators have been used in clinical assessments of those with neuromuscular disorders, although research on musculoskeletal disorders is limited.
Previous studies using evoked EMG as an indicator in tracking the recovery process up to sports resumption have investigated the neuromuscular function in the lower limb after ACL reconstruction. In such cases, even simple motor tasks may require increased the excitability of central nervous system function in the injured leg until the time for potential return.34 In this study, only the injured leg showed changes in the amplitude ratio (LLR/Mmax) due to differences in conditions, indicating that higher central nervous system involvement occurred in the injured leg, supporting the findings of previous studies. A study measuring latency, amplitude, and silent period duration from evoked EMG during finger motor tasks indicated that increased cortical excitability reflects the demands to perform tasks requiring greater precision.45 Furthermore, another study comparing LLR emergence characteristics across conditions of varying difficulty indicated that cortical function excitability related to the upper limbs might be higher when proprioceptive demand from the lower limb increases—under conditions of high motor control difficulty, requiring more conscious regulation, such as auditory tasks.39
Unlike previous studies, the current results showed that the amplitude ratio for the visual condition was significantly higher than that for the auditory condition in the injured leg. Additionally, LLR amplitude across conditions did not differ in athletes without a history of sports injuries. The differences from prior studies may be attributed to variations in the participants’ sports experience and history of lower limb injuries. Thus, future research should control for these factors by including participants with comparable physical characteristics and injury backgrounds. These results may indicate that the involvement of higher central nervous functions in athletes with a history of sports injuries was less under auditory conditions, with vision rather than proprioception predominantly regulating motor control, such as torque adjustment. Athlete with a history of sports injuries who has not returned to competition by the prescribed period showed increased mid-frequency of the torque curve during torque maintenance and decreased LLR/Mmax, indicating suboptimal fine muscle adjustments and reliance on rhythmic adjustments when relying on auditory cues.
Although athletes without a history of sports injuries showed no changes between the conditions, the excitability of central nervous system function increased when tasks were performed using vision in the injured leg among athletes with a history of sports injuries, indicating that motor guidance using visual feedback in the injured leg could lead to an increased likelihood of adjustments dominated by the excitability of central nervous system function rather than peripheral nervous functions, such as proprioception. Therefore, excessive use of visual feedback for movement guidance in the rehabilitation process may inhibit the automation (unconsciousness) of movements required in sports activities, requiring careful consideration of the feedback methods used in training.35
In addition to the assessment tools that have been used previously, the evaluation method employed in this study may serve as a useful aid in determining readiness for return to sport. However, this study had several limitations, including a small sample size and heterogeneity in the types of sports-related injuries. Moreover, due to the limited number of participants, it was not possible to analyze differences between the dominant and non-dominant limbs. Therefore, future studies should aim to increase the sample size and address these limitations to further advance this line of research.
CONCLUSION
The results of this study indicate that athletes with a history of sports injuries can achieve finer motor adjustments using visual feedback, facilitated by increased excitability of higher central nervous system functions. However, an over-reliance on visual feedback may persistently involve higher central nervous system functions, potentially hindering the automation (or unconscious execution) of movements.
Disclosures
This work was supported by the Special Research Grant from Hokuriku University for the 2024 academic year.
ACKNOWLEDGEMENTS
The authors would like to thank Hokuriku University for the support through the 2024 Special Research Grant.
.jpeg)
.jpeg)