INTRODUCTION
Infraspinatus atrophy (IA) is a frequently overlooked condition in professional tennis players and presents as a “hollowing or loss of soft tissue bulk inferior to the scapular spine in the infraspinous fossa,”1 visibly differing from the contralateral side. Despite noticeable muscle atrophy, IA typically does not result in pain or self-perceived functional deficits2; however, measurable deficits such as decreased external rotation strength, impaired shoulder proprioception, and abnormal scapulothoracic muscle recruitment patterns are often present.3–5 Given that the sensorimotor system, comprising both active and passive stabilizing structures, contributes to shoulder joint stability, atrophy of the infraspinatus may compromise athletic performance and predispose individuals to subsequent shoulder injuries. This clinical paradox—where athletes often report normal shoulder function despite underlying neuromuscular impairments—complicates diagnosis and allows the condition to remain undetected until preventative screenings are conducted.
The infraspinatus plays a key role in maintaining shoulder stability and facilitating efficient force transfer during tennis strokes.3 Adequate strength of the external rotator muscles is crucial during the cocking and acceleration phases of a tennis serve to generate power and protect the shoulder joint.6,7
Given its diagnostic challenges and the high prevalence of IA among overhead athletes,1,2,8 the aim of this clinical commentary is to highlight practical strategies for its early detection and management. By addressing IA even in the absence of symptoms, clinicians can safeguard athletes’ performance and attempt to reduce their risk of future injury.
EPIDEMIOLOGY
IA is often an early sign of an underlying issue, such as suprascapular nerve neuropathy (SNN), which causes muscle atrophy due to nerve impairment. SNN, which affects the supraspinatus and infraspinatus muscles, accounts for 1-2% of all shoulder pain and is frequently overlooked.9 It primarily affects individuals under the age of 40,8 with reported mean ages ranging from 28 to 38 years in athletic populations.10,11 Athletes who participte in overhead sports, including baseball, volleyball, and tennis, are particularly susceptible to SNN, as these sports place substantial loads on the shoulder in overhead, abducted, or externally rotated positions, increasing the risk of SNN and IA.12,13
Ellenbecker et al.1 observed 153 male professional tennis players during a musculoskeletal screening, including visual inspection of the infraspinous fossa. Around 92 athletes (60.1%) showed IA in the dominant arm, with a significant association found between IA and weaker external rotation strength. IA is also common in other overhead sports, such as volleyball and baseball. A systematic review by Challoumas and Dimitrakakis suggests that IA is related to repetitive ball impacts in overhead positions, with tennis and volleyball showing higher prevalence compared to baseball.12
Prevalence rates of IA appear similar between male and female tennis players. Young et al.2 reported a 52% incidence in female tennis players, while Ellenbecker et al.1 found 60% in male tennis players.1,2 Ferretti et al. observed equal incidence rates among genders, suggesting IA affects both male and female volleyball players similarly.13
Relevant Anatomy
The SN receives nerve fibers originate from the cervical spine nerve roots C5 and C6. It follows a deep path along the ventral surface of the upper trapezius and omohyoid muscle, then passes posterior to the clavicle beneath the insertion of the upper trapezius. The nerve traverses the suprascapular notch at the upper border of the scapula, giving off a branch to the supraspinatus. Continuing through the spinoglenoid notch, it courses inferior to the transverse scapular ligament and around the lateral border of the scapular spine to reach the infraspinous fossa, where it terminates. The spinoglenoid (inferior transverse scapular) ligament spans this notch, creating a tunnel through which the nerve travels.14–16
The SN primarily provides motor innervation to the supraspinatus and infraspinatus muscles of the rotator cuff. Additionally, its sensory branches serve the posterior aspects of both the glenohumeral and acromioclavicular joints. Sensation in the surrounding skin is supplied by the axillary nerve.14–16
Understanding the location and visualization of the scapular notches is crucial for comprehending the SN’s pathway and potential sites of entrapment. Figure 1 illustrates the suprascapular notch and the spinoglenoid notch, covered by the inferior transverse scapular ligament. The SN passes between these two notches, following the supraspinous fossa, where the supraspinatus muscle is also located.
Etiology
The symptoms and severity of IA depend on the lesion location. Lesions at the suprascapular notch affect both the supraspinatus and infraspinatus, while those at the spinoglenoid notch affect only the infraspinatus. The mechanisms of IA are broadly categorized into repetitive traction on the nerve and compressive forces. Research has proposed four theories to explain the high prevalence of IA among overhead athletes.9,12,17,18 These theories are presented from proximal to distal, rather than by their relative importance.
Theory 1: Suprascapular Nerve Entrapment (SNE) at the Suprascapular Notch Resulting in Suprascapular Nerve Neuropathy (SNN)
This theory suggests nerve compression or entrapment at the suprascapular notch. Holzgraefe et al.19 found 33% of high-level volleyball players had signs of SNN, with severe atrophy and weakness in external rotation (ER). Electromyography (EMG) confirmed loss of innervation in the infraspinatus in athletes with severe atrophy, and mild denervation in both infraspinatus and supraspinatus in others without clinical signs. This suggests that SNE likely occurs at the suprascapular notch, as both the supraspinatus and infraspinatus appear to be affected.19 Rengachary et al.20 proposed that nerve friction and constriction at the suprascapular notch contribute to SNE, relevant for both volleyball and tennis.
Theory 2: Suprascapular Nerve Neuropathy (SNN) at the Spinoglenoid Notch Due to Eccentric Infraspinatus Contraction
This theory examines how SNN may result from traction injuries during muscle activity. During the tennis serve, players perform a sequence of movements, including maximal ER in the cocking phase, followed by rapid acceleration and deceleration at ball impact. This places significant stress on the shoulder musculature, particularly the infraspinatus, which controls rotational movements of the shoulder joint. Ferretti et al. observed that maximal ER causes the infraspinatus contraction to shift the suprascapular nerve medially, stretching it and creating tension at the lateral edge of the scapular spine. At ball impact, the eccentric contraction of the infraspinatus further stretches the nerve. These eccentric contractions and deceleration forces strain the nerve at the spinoglenoid notch.13,21
Theory 3: Suprascapular Nerve Neuropathy (SNN) at the Spinoglenoid Notch Due to Extreme Shoulder Positions
This theory addresses traction injuries from extreme shoulder positions frequently encountered by overhead athletes. Reeser et al.22 highlighted that shoulder abduction and horizontal adduction during volleyball serves lead to scapular rotation, elongating the suprascapular nerve. The resulting shear stress can damage the nerve, especially at the spinoglenoid notch, leading to IA.22 Figure 2 depicts a tennis player demonstrating the extreme shoulder positions reached during a serve motion.
Theory 4: Compression of the Suprascapular Nerve Due to Posterior Shoulder Tightness
This theory proposes that repetitive activation and resulting tightness in the posterior shoulder may lead to compression of the suprascapular nerve. Repetitive overhead motions, like serving, lead to increased external rotation in the maximally abducted position, which is essential for generating high throwing velocities.23 To counteract and decelerate the high forces generated during the tennis serve and forehand groundstrokes, the posterior rotator cuff muscles (infraspinatus and teres minor) and scapular stabilizers are heavily activated during the follow-through phase. Often this results in localized tightness of the posterior capsule as well as the infraspinatus and teres minor muscles.24 This tightness, described as thixotropy,25,26 may contribute to suprascapular nerve compression during shoulder adduction and internal rotation, especially in the follow-through position.1 Plancher et al.27 suggested that the spinoglenoid ligament, which blends with and inserts into the posterior rotator cuff, may further contribute to this compression at the spinoglenoid notch. These effects may be aggravated by joint positions such as scapular protraction and glenohumeral internal rotation—which commonly occur during the follow-through phase of a forehand or serve—and place additional mechanical pressure on the nerve by reducing the available space within the notch.28
Over time, this posterior shoulder tightness can contribute to a glenohumeral internal rotation deficit (GIRD), which refers to a loss of internal rotation in the dominant shoulder of more than 20° compared to the non-dominant side.25 GIRD is commonly observed in overhead athletes and is thought to result from adaptations in the posterior capsule and musculature due to repetitive loading.29 While GIRD itself may not directly compress the suprascapular nerve, it reflects the underlying soft tissue tightness that can narrow the spinoglenoid notch and limit normal scapulohumeral motion, thereby increasing the risk of nerve entrapment during specific phases of the throwing or serving motion.
History Taking
IA often develops gradually without a history of acute injury, and athletes may experience painless atrophy, particularly of the infraspinatus, leading to weakness during sport-specific activities. The extent of this weakness depends on the severity of the atrophy and the compensatory action of the teres minor.2,8,9,30 Asking about an athlete’s involvement in overhead sports and their shoulder function is crucial, including details such as the type of sport, frequency and intensity of activity, history of pain or instability, and any limitations in performance or range of motion.
Though IA itself may not cause pain, SNE—especially at the suprascapular notch—can. If pain is present, a thorough history including symptom onset, trauma, and prior treatments should be taken. Athletes often describe vague, deep, dull posterior shoulder pain, which can radiate and worsen with overhead activity.8,30
In addition, clinicians should remain alert for red flags that may indicate more serious pathology beyond SNE. These include acute onset of shoulder weakness, non-mechanical deep shoulder pain, neurological signs inconsistent with isolated rotator cuff dysfunction, and clinical indicators of malignancy such as unexplained masses, night pain, rapid weight loss, or a known history of cancer. Systemic symptoms such as fever, night sweats, or progressive pain unrelated to activity should also prompt further investigation.31
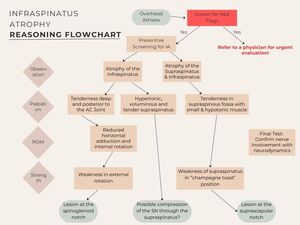
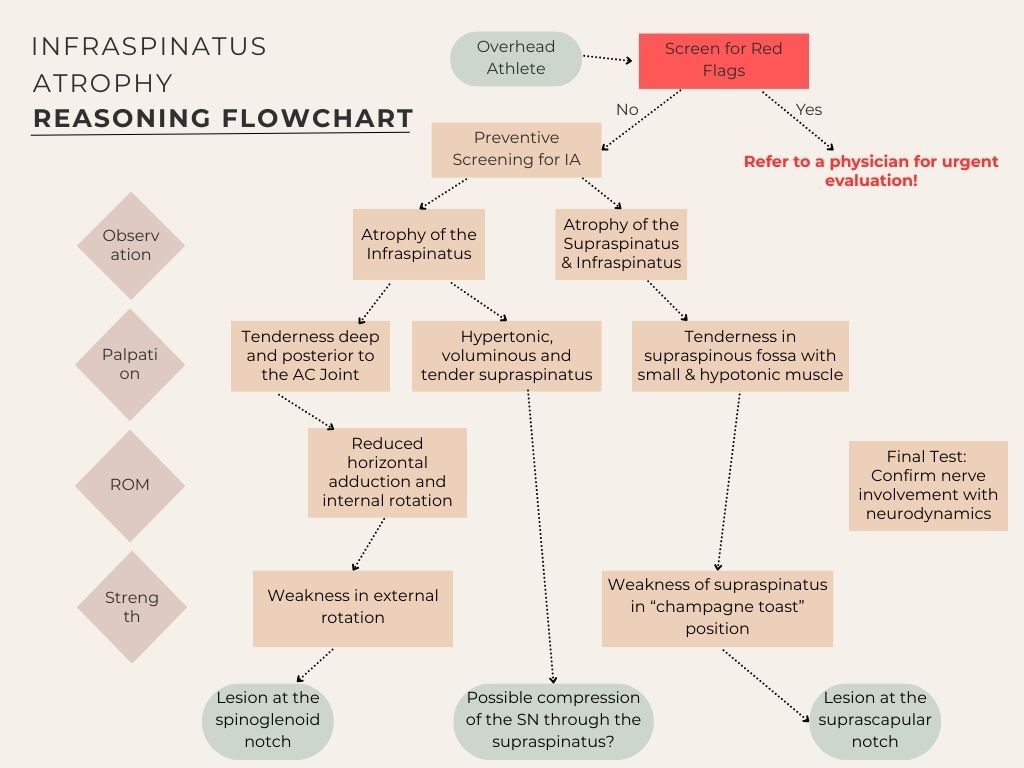
Figure 3 summarizes the screening process for IA, outlining assessment steps and the aetiology that may lead to different forms of IA. This helps guide clinicians in identifying potential nerve lesions and their anatomical locations.
Assessment: Clinical Reasoning for Identifying IA
A detailed clinical examination is key to confirm the diagnosis and assess any underlying causes.
Observation
IA can be visually assessed from a posterior view with the athlete standing (feet together, arms at sides, thumbs on hips pointing backward) or in a sitting position, as shown in Figure 4. A simple “yes/no” classification is sufficient. However, observation is subjective, and well-developed shoulder muscles in tennis players may mask true IA. To minimize misdiagnosis, clinicians should supplement visual assessments with objective methods like imaging or functional testing.
Atrophy of the supraspinatus should also be evaluated to rule out SNE at the suprascapular notch. This involves inspecting the superior shoulder borders from behind for bilateral asymmetry.30 Diagnosing supraspinatus atrophy can be challenging due to the overlying bulk of the upper trapezius. An example of a tennis player with IA can be seen in Figure 4.
Palpation
Palpation assessment should include evaluation of tissue quality (e.g., pliability, tone, and texture) to help differentiate between normal, adaptive, and pathological states. Clinicians should systematically compare bilateral structures-such as the supraspinatus and infraspinatus fossae - for symmetry, tenderness, atrophy, or hypertrophy. In cases of SNE, the affected side may demonstrate increased tissue firmness due to fibrosis or muscle guarding, or conversely, a soft, atrophic quality in cases of chronic denervation. Pliability is best assessed through gentle, sustained manual compression and rolling of the superficial and deeper tissues to evaluate mobility, resistance, and elasticity. A healthy muscle typically offers mild resistance with some give, whereas increased stiffness, adhesions, or decreased glide may indicate chronic irritation, scarring, or neural involvement.30,32,33
Testing ROM
ROM assessments are essential for identifying joint mobility limitations, aiding treatment planning and progress tracking. GIRD should be evaluated as a potential factor in IA. The spinoglenoid ligament, connecting the scapular spine, glenoid neck, and posterior shoulder capsule, may tighten during cross-body adduction and internal rotation, potentially resulting in dynamic nerve compression and pain at the spinoglenoid notch.8,30 Such movements may show ROM limitations. Research indicates that dominant shoulders in tennis players typically have reduced internal rotation ROM (approx. 5-10°) and reduced total rotation compared to the nondominant side.34 Scapular stabilization during testing is crucial to avoid compensatory movements that obscure internal rotation and horizontal adduction limitations.1,35 These assessments should be performed with the patient in a supine position, where one hand stabilizes the scapula while the other guides the arm into internal rotation or horizontal adduction. Conversely, external rotation ROM is often greater in the dominant shoulder due to the extreme positions required in tennis.36
Isometric Strength Testing
Strength assessment is a critical component in evaluating IA, and multiple testing approaches may provide complementary insights. Isometric strength testing using a hand-held dynamometer is a reliable method, typically performed with the device positioned proximal to the wrist. This is often done using a “make” test, in which the examiner holds the dynamometer stationary while the patient gradually builds up and maintains maximal voluntary force against it—hence the term “make.” In contrast, a “break” test involves the examiner applying force until the patient’s limb gives way, offering insight into eccentric strength. To ensure safety, especially in patients with suspected neuropathy or muscle weakness, the examiner should apply force in a gradual, controlled manner to minimize the risk of discomfort or injury. For greater functional relevance, break testing can be performed at different arm positions—such as 0° and 90° of abduction—to assess eccentric control of the posterior rotator cuff, a key demand during the deceleration phase of the tennis serve.37
Testing external rotation at 0° abduction is commonly used to assess infraspinatus strength, as it emphasizes its contribution more than at 90° abduction, where the teres minor may be more involved.1 Assessment of the supraspinatus can support identification of the location of SNE. The “champagne toast” position (30° abduction, mild external rotation) is often preferred, as it may better emphasize supraspinatus activation while reducing deltoid involvement compared to the traditional “empty can” test.38
The highest measurement from two trials in kilogram-force (kgf) is recorded and compared to the unaffected side to identify strength deficits. A strength loss greater than 10% may be considered significant, as demonstrated by Ellenbecker et al., who reported a mean external rotation strength deficit of approximately 12.2% in athletes with IA.1
Neurodynamic Testing
To assess for potential SNE, it is crucial to evaluate the mobility and gliding of the SN. Effective methods for this evaluation include the suprascapular nerve stretch test and the cross-body adduction test. These tests can help identify any restrictions or pain associated with nerve compression, providing valuable insights for diagnosis and treatment planning.
Suprascapular Nerve Stretch Test: The examiner stands behind the seated patient, side-bending and rotating the head away from the affected shoulder while gently protracting the shoulder. A positive test is indicated when the maneuver increases pain at the posterolateral shoulder.4,8,30 An image of this test can be seen in the Appendix as Figure 5.
Cross-Body Test: With the patient in a supine position, the examiner stands on the side opposite the shoulder being tested. The test begins by passively side-bending the patient’s cervical spine away from the affected shoulder to place tension on the neural structures. Subsequently, the examiner horizontally adducts the patient’s arm across the body, applying gentle pressure over the posterior aspect of the glenohumeral joint to maintain the position. Simultaneously, the examiner facilitates scapular protraction by applying anterior pressure on the medial border of the scapula. The test is considered positive if this maneuver reproduces the patient’s posterior shoulder pain or neurologic symptoms, suggesting suprascapular nerve irritation. For visual reference, please see Figure 6 in the Appendix.8,30
Passive Testing for Joint Dysfunction (Tightness & Laxity)
The shoulder joint should be evaluated for glenohumeral instability, including both inferior and anterior. In cases of joint instability, the infraspinatus may compensate to stabilize the glenohumeral head within the glenoid fossa. This increased demand on dynamic stability can lead to overstrain and overactivity of the infraspinatus, contributing to tightness and subsequent nerve compression over time. Inferior instability can be assessed using the sulcus sign test, while anterior instability is evaluated with the apprehension and relocation tests. Screening for micro-traumatic posterior shoulder instability is also important, as it is often overlooked in tennis players. Symptoms typically occur in positions of flexion, horizontal adduction, and internal rotation. Posterior capsulolabral instability increases humeral head translation.39–41
Testing Scapular Dynamics and Stability
Leider et al. describe scapular instability as a potential source of IA. A weak or unstable scapula may cause compression of the SN as it passes through the suprascapular notch. Therefore, coordination in various scapular movements is crucial.30
Scapular muscle testing should include both isolated and functional assessments. Functionally, clinicians can observe scapular dynamics—such as retraction, protraction, elevation, depression, and upward or downward rotation—during active arm abduction. Isolated testing of the middle and lower trapezius can be performed in prone using the “T” and “Y” positions, while the serratus anterior can be assessed through exercises such as the quadruped serratus press or foam roller wall slides. Adding a loop band around the wrists during wall slides may help simulate rotator cuff co-contraction, potentially revealing hidden weaknesses (Appendix, Figure 7). Clinicians should evaluate movement quality, compensatory strategies, and the strength and endurance needed to maintain each test position. Table 1 which outlines the key muscles contributing to each scapular movement is provided below.
Differential Diagnostics
To differentiate suprascapular nerve entrapment (SNE) from other causes of posterior shoulder pain or weakness, a thorough clinical examination and patient history are essential. Particular attention should be given to the onset of symptoms—whether sudden or gradual—and any history of trauma, repetitive overhead activity, or prior cervical or shoulder pathology.
Differential diagnosis should include cervical disc herniation or radiculopathy, rotator cuff tears or tendinopathies, subscapular edema, subacromial pain syndrome, and SLAP (superior labrum anterior to posterior) lesions. These conditions can mimic or contribute to suprascapular nerve symptoms and should be ruled out through targeted clinical tests (e.g., Spurling’s for cervical radiculopathy, “Champagne toast” (as described above) test for supraspinatus integrity, O’Brien test for SLAP lesions) and confirmed by imaging as appropriate. Depending on the pathology, ultrasound or MRI can help detect space-occupying lesions such as cysts or tumors. In particular, paralabral cysts associated with labral or capsular tears may compress the suprascapular nerve along its course over the scapula.
For more precise nerve evaluation, electromyography (EMG) may aid in confirming suprascapular nerve involvement and in ruling out more proximal causes such as cervical nerve root compression or other upper trunk neuropathies.8,9,18
Problem Solving, Rehabilitation, Treatment, and Prevention of Infraspinatus Atrophy
The treatment of IA should focus on addressing atrophy, stimulating the infraspinatus, and preventing further nerve damage. Thus, strengthening the external rotators, especially the infraspinatus, is vital, even without pain or functional loss. Rehabilitation should focus on muscle activation and gradual strengthening to restore shoulder stability and prevent progression of the atrophy. Athletes with no functional limitations or pain may continue their sport with modified training loads to avoid exacerbating the condition. One approach is to monitor weekly load progression using the acute:chronic workload ratio (ACWR), calculated by dividing the current week’s training load (e.g., total duration × session RPE) by the average load of the previous three to four weeks. A ratio between 0.8 and 1.3 is generally considered to be within a safe range, while values above 1.5 have been associated with a higher risk of injury.42
For symptomatic cases, non-operative treatment is preferred initially, including physical therapy, non-steroidal anti-inflammatory medications (NSAIDs), and training modifications. Monitoring the response to conservative treatment is critical, as prolonged nerve compression could lead to irreversible muscle atrophy. While clinical improvement may occur within months, visible reversal of infraspinatus atrophy is not commonly reported and difficult to monitor without imaging; thus, treatment progress is typically evaluated through functional outcomes rather than muscle bulk. Conservative management is often effective, with most patients responding within six months. If pain reduces and function improves, conservative treatment should continue for up to 12 months. If symptoms persist after three months, minimally invasive treatments may be considered. For cases with a rotator cuff tear, addressing the primary issue is preferred over isolated nerve decompression.17,30
Even without a formal SNN diagnosis, IA patients can benefit from rehabilitation strategies used for rotator cuff tendinopathy. Athletes with symptoms should avoid movements that trigger discomfort, particularly during the early or acute phase, which can vary between individuals. Activities like reaching backward, overhead motions, and tennis serves should be limited initially to prevent small injuries from worsening. As symptoms subside and shoulder function improves, these activities can be gradually reintroduced.
Key Aspects of Conservative Treatment
Education
Athletes with symptoms should avoid movements that trigger discomfort, particularly during the early or acute phase, which can vary between individuals. Activities like reaching backward, overhead motions, and tennis serves should be limited initially to prevent small injuries from worsening. As symptoms subside and shoulder function improves, these activities can be gradually reintroduced.
Sleeping on the unaffected side with the affected arm supported on a pillow can help reduce symptoms and prevent overstretching of the infraspinatus and SN. The head should remain in a neutral position to avoid morning stiffness.5 This position is illustrated in Figure 8 in the Appendix.
Preventing Stiffness of the Posterior Capsule and the Infraspinatus
Preventing stiffness in the posterior capsule and musculature is important to reduce the risk of SN compression, particularly when the shoulder is in adduction and internal rotation. Implementing preventive measures can help mitigate this risk. Some effective examples include:
Cross-Body Adduction Stretch: Performed by pulling the affected arm across the body while keeping the scapula retracted. Optionally, it can be done under a warm shower.5 This stretch targets posterior capsule tightness, which is often associated with GIRD and increased risk of suprascapular nerve irritation. While this position resembles that used in diagnostic testing, the goal here is gentle mobilization, not provocation of symptoms. Unlike the cross-body adduction neurodynamic test, which places the scapula in protraction to increase tension on the suprascapular nerve, the therapeutic stretch is performed with the scapula stabilized in retraction. This likely reduces neural loading and selectively targets the posterior capsule and external rotators, minimizing the risk of neural irritation. The stretch is illustrated in Figure 9 in the Appendix.
Sleeper Stretch: While lying on the affected side with the shoulder and elbow flexed to 90°, the individual uses the opposite hand to gently press the forearm toward the table, internally rotating the shoulder. The stretch should be held for 30 seconds and repeated 3 times, ideally once or twice daily, ensuring no sharp pain is felt. A visual reference for this stretch can be found in Figure 10 in the Appendix.
Post-Isometric Relaxation (Contract-Relax) with Respiratory Augmentation and Shoulder Rotation: While lying in supine, slow, deep breaths are encouraged as the arm falls into internal rotation. Voluntary internal rotation is also encouraged to further stretch the external rotators while reciprocally inhibiting them.5 This technique is shown in Figure 11 in the Appendix.
Neurodynamics (Nerve Gliding)
Neurodynamic exercises focus on gently gliding nerves along their pathways to relieve compression and enhance function. These controlled movements aim to alleviate symptoms and improve nerve mobility without inducing pain, numbness, or tingling. The objective is to support healing and facilitate effective rehabilitation while avoiding symptom exacerbation.
Suprascapular Nerve Glides (Active Without Therapist): The affected person sits up straight, retracts the shoulders, and tucks the chin. They then side-bend toward the unaffected side, raise the affected arm overhead, and bend the elbow, repeating with steady breathing. An illustration of this nerve glide technique can be seen in the Appendix in Figures 12 and 13.
Strengthening of the Rotator Cuff Muscles
The goal of a strength exercise program for athletes with IA is to progressively improve rotator cuff strength. The program should start with isolated concentric and eccentric exercises and gradually progress to more complex, functional overhead and plyometric movements. Emphasizing sport-specific movements and controlled loading of the infraspinatus and surrounding muscles is key. Given that SNN caused by eccentric infraspinatus contractions can lead to IA, incorporating controlled eccentric exercises can improve strength and functionality while preventing overuse injuries from high-impact forces during serves and forehands.
Athletes with IA should be referred to an athletic trainer or physical therapist for a comprehensive assessment of strength, mobility, and function. Based on this, a tailored rehabilitation program should be developed, incorporating exercises across various planes and intensities.
Scapular Control and Strengthening
Effective control of scapular movements, such as elevation, protraction, retraction, and depression, is crucial for shoulder health and recovery from IA. Strengthening the scapulothoracic muscles helps alleviate symptoms and prevent future damage.30 A study by Contemori and Biscarini43 found that during shoulder abduction in the scapular plane, athletes with isolated infraspinatus atrophy showed significantly higher activity in the deltoid and upper trapezius, along with delayed activation of the serratus anterior, compared to their contralateral shoulder and healthy athletes. These findings underscore the importance of targeting the serratus anterior, as well as the middle and lower trapezius, in scapular strengthening programs to restore balanced muscle activation and improve scapulothoracic control.
Scapular control exercises should begin with basic movements and progress to functional plyometric drills—such as medicine ball throws and overhead serving simulations—first using a resistance band, then a racket, to safely develop coordination, strength, and shoulder stability.
Manual Treatment
To address IA, specialists should use passive mobilization techniques targeting areas like the suprascapular notch, spinoglenoid notch, and supraspinatus. These techniques aim to relieve nerve compression. Evidence suggests that passive joint mobilization can quickly improve muscle function.44 Below, the authors describe a direct mobilization of the suprascapular notch, an indirect mobilization of the spinoglenoid notch and a release of the supraspinatus and upper trapezius in prone position. After treatment, neurodynamic tests like the suprascapular nerve stretch or cross-body test can assess the effectiveness of the techniques.
-
Direct Mobilization of the Suprascapular Notch: The affected person lies on their unaffected side while the therapist positions their hands around the upper scapular region, applying pressure with their fingers focused near the suprascapular notch. To access this area effectively, the therapist must gently shift the trapezius to the side. Since this region contains the SN, releasing tension or adhesions here can enhance its function by creating a more favorable environment. Please refer to Figure 14 in the Appendix for illustration.
-
Indirect Mobilization of the Spinoglenoid Notch: With the affected person in a side-lying position, the therapist’s thumb focuses on the spinoglenoid notch. Upon identifying an area of tension, the shoulder is passively moved into external rotation to facilitate a release and improve mobility in the region. This technique is demonstrated in Figure 15 in the Appendix.
-
Release of the Supraspinatus and Upper Trapezius in Prone Position: The therapist’s fingers are positioned beneath the upper trapezius and supraspinatus muscles near the supraspinous fossa. Targeted pressure is applied to release tension in these muscles (figure 16 in the appendix). This technique aims to reduce pressure on the suprascapular nerve by alleviating muscular tightness and enhancing the surrounding tissue environment.
Soft Tissue Techniques
Self-care techniques, such as using a fascial ball for soft tissue release, can help manage IA by reducing muscle stiffness and increasing ROM. These should be combined with dynamic stretching and an active warm-up before training. Foam rolling the infraspinatus and surrounding muscles alleviates tightness and improves tissue pliability. Regular self-massage can reduce muscle stiffness and prevent nerve compression, supporting muscle function and recovery.45
Dry Needling
In addition, dry needling has emerged as a targeted intervention for addressing neuromuscular dysfunction, particularly in cases involving hypertonicity of the supraspinatus or infraspinatus. This technique can help reduce trigger point activity, improve muscle activation patterns, and support overall rehabilitation when applied by a qualified practitioner. Evidence suggests that dry needling can produce lasting analgesic effects in patients with shoulder pain, as shown in a double-blind, sham-controlled trial where pain relief persisted for at least seven days post-treatment.46 Furthermore, a case report demonstrated that dry needling increased infraspinatus muscle thickness and external rotation strength in a subject with persistent muscle dysfunction, indicating its potential to support muscle recovery and hypertrophy in clinical scenarios involving infraspinatus atrophy.47
Prevention
To reduce the likelihood of IA and shoulder injuries, preventive strategies similar to those used in treatment should be implemented. Given the high prevalence of IA among youth and early adult tennis players, incorporating preventive measures into warm-up routines is essential.1 These strategies should be consistently practiced to effectively address injury prevention. Emphasizing early intervention will protect against IA, enhance performance, and improve musculoskeletal health. Preventive measures should focus on maintaining full passive and active ROM (including posterior capsule stretching), gradually strengthening external rotators and periscapular muscles (especially loading the infraspinatus eccentrically overhead with scapular control), nerve flossing of the SN, and specific manual therapy to release surrounding structures in a step-by-step approach.
Challoumas & Dimitrakakis proposed that SNN at the spinoglenoid notch may result from traction injuries, where elongation and traction cause shear stress, potentially damaging the nerve and leading to atrophy.12 This highlights the importance of incorporating regular nerve gliding exercises into the training regimen of athletes prone to IA. These exercises promote nerve mobility, reduce the risk of entrapment and compression, and help prevent muscle atrophy related to neuropathy.
Prognosis
According to current literature, IA typically responds well to conservative treatment programs. In cases where IA is associated with structural issues, such as ganglion cysts, surgical intervention may be necessary.48,49 Generally, athletes can return to their previous level of sports participation following appropriate therapeutic interventions.
Discussion
This article provides a practical approach to diagnosing and managing IA in tennis players. IA is prevalent among athletes, highlighting the need for regular screenings to detect it early, especially during training periods with high volumes of serving. Early detection helps prevent long-term issues, structural changes, and potential injuries in the kinetic chain. The assessment should involve reviewing the athlete’s history, visual inspection, palpation, and functional tests. Given the infraspinatus’ crucial role in shoulder biomechanics and the serve motion, timely identification and management of IA are essential for optimal performance and injury prevention.
Ellenbecker et al.1 identified IA using visual inspection, but this subjective method raises concerns about its reliability. To improve objectivity, quantifying the bilateral thickness of the infraspinatus via musculoskeletal ultrasound (MSK-US) is recommended. This technique complements visual observation by providing measurable data and accounting for potential hypertrophy in surrounding musculature that may mask IA.1 However, standardized cut-off values for infraspinatus thickness have not yet been established, highlighting the need for further research to refine diagnostic accuracy and clinical decision-making.
Further research, particularly using rehabilitation ultrasound for diagnosis, is needed to provide more accurate insights into the extent and nature of IA, improving diagnostic precision.
IA is commonly seen in tennis players who transition too quickly into explosive activities, such as serving a high number of balls, without adequate preparatory phases and gradual training buildup. This rapid escalation and overload of the infraspinatus can lead to IA, especially in young athletes whose shoulder musculature may not yet be fully developed to handle such loads. Contemori et al.50 highlighted altered scapulothoracic muscle imbalances in professional volleyball players with IA, emphasizing the importance of proper muscular development of the shoulder girdle for injury prevention. Similarly, Gibson et al.51 identified key risk factors for shoulder injury in youth athletes, including imbalances in rotator cuff strength, range of motion deficits, and insufficient sport-specific preparation—particularly in athletes exposed to high loads during competition. These findings underscore the need for gradual progression in training load, especially in young tennis players.
Young players often engage in low-load shoulder exercises in neutral positions, such as external rotator training with a light resistance band. However, this does not adequately prepare the shoulder for the high loads of serving. Pre-participation training should have a preventive focus, strengthening overhead athletes step-by-step for high-load actions before they experience explosive forces. Athletic trainers and physical therapists should establish structured progressions to determine when a player is ready for high-volume serving. A significant gap often exists between low-load exercises and the high-load demands of serving, especially in youth players. The mean age of tennis players in the study from Ellenbecker et al. study was around 25 years, which likely reflects years of inadequate sport-specific shoulder conditioning.1
A slow, gradual increase in training intensity, emphasizing prevention, muscle conditioning, and controlled movements, can help mitigate the risk of IA. A structured and progressive training regimen can significantly reduce the likelihood of shoulder injuries and atrophy.
The role of the supraspinatus in relation to suprascapular nerve entrapment (SNE) in overhead sports remains underexplored. Anatomically, the supraspinatus lies superior to the suprascapular nerve (SN) as it travels from the suprascapular notch beneath the superior transverse scapular ligament to the spinoglenoid notch.15 In tennis players, changes in the supraspinatus—such as hypertrophy, overuse, or tendon rubbing—are frequently observed in the dominant arm and may influence the SN, potentially contributing to IA. The compression forces generated during the serve reduce the space between the supraspinatus and the SN, which supports the author opinion that the supraspinatus may contribute to SNE and IA. A similar concept was proposed by Sandow and Ilic who emphasized dynamic compression during the cocking phase of the serve.52 In that phase, shoulder abduction and external rotation cause the supraspinatus to contract and draw the tendinous margin between the supraspinatus and infraspinatus tendons against the lateral scapular spine, compressing the infraspinatus branch of the SN at the spinoglenoid notch.7 In contrast, the author opinion presented here suggests that a hypertrophic and hypertonic supraspinatus in tennis players could exert more constant pressure on the SN within the supraspinous fossa. Further investigation is needed to clarify the supraspinatus’ compressive role in SNE.
Looking at the proposed theories of aetiology, three out of four suggest isolated atrophy of the infraspinatus, which is also commonly observed in practice. In contrast to the infraspinatus, the supraspinatus rarely exhibits atrophy in tennis players. Therefore, the authors hypothesize that IA results from a combination of SNN at the spinoglenoid notch due to eccentric infraspinatus contractions, extreme shoulder positions, and compression from posterior muscular and capsular structures. Further cross-sectional studies are needed to confirm the rarity of supraspinatus atrophy in tennis players and to better understand the underlying aetiology of this condition.
Young et al.2 found no functional deficits or associations with concurrent shoulder disorders. Similarly, Strauss et al.18 observed high rates of compressive neuropathies and significant muscle atrophy in overhead athletes, but most did not show clinically relevant functional deficiencies, highlighting a paradox. This may be due to compensatory mechanisms in other rotator cuff muscles and surrounding musculature. The infraspinatus weakness is often compensated by other muscles, such as the teres minor, which can take on additional load to maintain shoulder function.53 As a result, athletes can maintain normal biomechanics, allowing for overhead movements without noticeable impairments. However, this imbalance can lead to disruptions in the kinetic chain, potentially causing pain or functional loss in other areas, like the elbow or spine, as compensation occurs. Elite athletes may also rely on advanced neuromuscular control and proprioception to adjust biomechanics subtly and preserve performance.2,18,53
Although IA may not directly correlate with shoulder injuries or functional deficits, the limitations athletes experience poses a risk for future issues. Therefore, detecting IA should be viewed as critical, and a potential warning sign. The studies by Young et al.2 and Strauss et al.18 focus on the impact of IA on shoulder injuries, but the effects of IA on other parts of the functional chain remain unclear.
Future research should include longitudinal studies to track the progression of IA and its effects on shoulder function and performance. Comparative studies are also needed to identify the most effective preventive and rehabilitative exercise programs. Lastly, further investigation is required to explore the correlation between IA and performance metrics, such as serve speed in tennis players.
Conclusion
IA is a common but often overlooked condition in overhead athletes, especially elite tennis players. This clinical commentary emphasizes the importance of regular preventative screenings to detect IA early and initiate an appropriate exercise program, which includes the reduction of potential risk factors. Implementing these strategies can significantly reduce the incidence and impact of IA, helping athletes remain healthy and competitive while enhancing their overall musculoskeletal health and performance.
Even if a tennis player does not exhibit functional deficits, it remains crucial to frequently assess for IA to prevent injuries elsewhere in the body or to identify the source of nearby injuries. Coaches, physical therapists, and athletes across all overhead sports should focus on specifically strengthening the external rotators in overhead positions and address shoulder imbalances.
Conflicts of Interest
The authors declare no conflicts of interest.