INTRODUCTION
Osteoarthritis (OA) is an increasingly prevalent disease, leading to disabling pain, functional impairment, and deformity. In 2019, OA affected 527.8 million people worldwide, with 41.5 million incident cases, an almost 50% increase since 1990.1 The number of people with symptomatic OA is proportionally higher in countries with developed market economies and rising rates of obesity, such as the United States. There is also a substantial economic burden placed on health systems due to greater utilization of government sponsored services and years of work lost. This strain is only anticipated to grow as the American population ages.2–4 Reduced health-related quality of life including impact on mental health, general health, and sleep are widely recognized sequalae associated with pain and loss of physical activity in persons with OA.2,4,5
When focusing on the shoulder joint, it is difficult to ascertain the true epidemiology of glenohumeral OA (GHOA) in the United States due to lack of population-based reports. Studies performed within the past two decades estimate a wide range of prevalence, from 5-21%6–8 depending on geographic population. Despite the limited understanding of GHOA prevalence, the rising number of shoulder arthroplasties suggests an increase in symptomatic disease.9–11
Options for management of GHOA continue to evolve as the complex pathogenesis becomes better understood. Degeneration of the interfacing articular cartilage and underlying subchondral bone, along with inflammation, scarring, and thickening of the richly innervated synovial capsule and surrounding soft tissue, act as noxious stimuli and contribute to mechanical limitations.3,12
Conventional conservative treatments are categorized into non-pharmacologic and pharmacologic modalities, often used synchronously to achieve maximal benefit.13–15 A widely accepted clinical recommendation includes a well-planned, structured strengthening and range of motion (ROM) program implemented under therapist guidance. In combination with systemic medications or intra-articular injections to disrupt the inflammatory process, improvements in symptoms and function can be achieved.16
However, with refractory disease, patients and their care team are left to consider alternative pathways. Orthobiologic joint injections including platelet-rich plasma, bone marrow aspirate concentrate (BMAC), and micronized fragmented adipose tissue (mFAT) have promising preliminary outcomes.17,18 Due to inconsistency of injectate compositions and remittance that often falls outside of insurance-payer coverage, they may be considered less desirable options.
The brisement procedure, or articular capsule hydrodilatation with an injectate composed of a steroid or anti-inflammatory, local anesthetic, and normal saline or sterile water, has potential to fill the gap as an affordable, low risk alternative which effectively targets OA pain while simultaneously improving ROM. Brisement has been widely applied for the successful treatment of adhesive capsulitis, with superior outcomes when performed in close temporal proximity to mobility interventions by a physical therapist (PT).19,20 Mechanistically, the administration of intra-articular steroid paired with disruption of synovial adhesions and capsular thickening, targets pathologic pathways identified in the development of GHOA.21 A team-based application of the brisement procedure, consisting of injection and immediate therapy, is proposed within this commentary as a novel, effective, accessible, and low-risk option for treatment of GHOA in the carefully selected patient. The purpose of this clinical commentary is to introduce a novel treatment approach combining brisement, an ultrasound-guided hydrodilatation procedure, with a structured physical therapy program to manage GHOA.
PATHOPHYSIOLOGY OF GHOA AND RATIONALE FOR BRISEMENT WITH PHYSICAL THERAPY
The pathogenesis of OA is complex with cellular and biochemical alterations within the cartilage, bone, synovium, and surrounding soft tissue resulting in joint dysfunction.12 Cartilage erosion leads to subchondral damage and osteophytic growth. Changes to the synovium are present in both early- and late-stage osteoarthritic disease. Several distinct synovial phenotypes have been identified, characterized by varying degrees of vascularity, inflammatory mediators, hyperplasia, fibrin deposition, and macrophage infiltration. These biologically active metabolites contribute to inflammation, fibrosis and ultimately, mobility restriction.21,22 Osteophyte formation and capsular thickening may also be seen in certain patterns of glenoid wear as a compensatory mechanism to splint or immobilize the painful glenohumeral joint (GHJ).23
Benefits of joint hydrodilatation for management of osteoarthritis have been described as early as 1984. In a triple-blinded RCT of hip capsular distention for OA, pain relief and improvements in ROM were observed for 12 weeks after the procedure.24 While the mechanism for symptomatic improvement is currently unknown, it can be theorized that the physical disruption of the restrictive fibrous tissue along with the modulation of synovium-generated inflammatory processes through volume-mediated capsular stretch and anti-inflammatory injectate, contribute to improved pain and function. To the authors’ knowledge, hydrodilatation of the GHJ has not been reported for management of symptomatic OA.
Ultrasound guided suprascapular nerve blocks have been utilized in hydrodilatation procedures for adhesive capsulitis due to the nerve’s role in sensory innervation of the GHJ and its accessibility via ultrasound guidance. The decision for regional analgesia is made to improve patient tolerance of the large volume subsequent injection and immediate manipulation.19
The glenohumeral musculature and non-contractile soft tissue surrounding the GHJ are important stabilizers of the humeral head within the glenoid. They also act as key mobilizers, dependent on strength and flexibility. With GHOA, muscular and soft tissue imbalances cause alterations in kinematics which further contribute to dysfunction.25 Glenoid wear patterns may result in rotation, translation, or subluxation of the humeral head. Changes in muscular recruitment to maintain anatomic movement or avoid painful movement lead to muscle atrophy and soft tissue contracture.26 As outlined in this commentary, the physical therapy program for GHOA used in conjunction with brisement targets these liable tissues. Identification of tonic musculature and management with joint, scapular, and thoracic mobilization is followed by stretching and eventual strengthening. Addressing these external constraints of the GHJ by the PT are critically important for achieving maximal outcomes in ROM after brisement. The role of the PT is not only to address post procedure ROM and function but to identify those patient-specific factors that contributed to development of, or perpetuated, the problem once developed.
PATIENT SELECTION
GHOA has a presentation that, like many arthritic conditions, has a variety of unique features. In selection of a patient who may be a candidate for the brisement procedure, there are several important clinical considerations:
- Significant glenohumeral degeneration is not an exclusion; however, the procedure should be avoided in patients with concomitant severe cuff arthropathy and those that have more than minimal posterior rim erosion (Type B glenoid wear, Table 1).
-
Avoidance in patients with active cervical radiculopathy, due to the potential inhibition of GHJ mobility.
-
Avoidance in patients with significant rotator cuff weakness. Ability to actively move the shoulder is required for efficacy of the physical therapy program.
-
Avoidance in patients who have had rotator cuff surgery in the prior six months. The procedure can be considered based upon discussion and clearance with the performing surgeon.
A unique consideration for the application of the brisement procedure in patients with osteoarthritis, as compared to those with adhesive capsulitis, is the potential for bony joint mobility restrictions related to the arthritic process. For this reason, it is recommended to have plain films performed and reviewed by the treating physician at a reasonable interval prior to the brisement procedure. Discomfort should be openly communicated between the patient and therapist during all hands-on treatment.
DESCRIPTION OF BRISEMENT PROCEDURE
Suprascapular Nerve Block
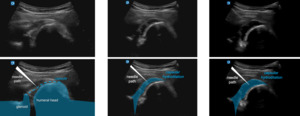
First, a suprascapular nerve injection either at the suprascapular notch or at the spinoglenoid notch is performed. This nerve block allows the patient to better tolerate the large volume second injection as well as the post-procedural manual stretching. Typically, a posterior musculoskeletal ultrasound (MSK-US) approach is utilized (Figure 1) to inject 3-5 cc of 1% lidocaine under the transverse ligament surrounding the notch.
Posterior GHJ Injection
Next, the GHJ is visualized and injected via MSK-US from an in-plane medial to lateral approach. Once needle placement is confirmed, 20-30 cc total injectate is administered (Figure 2). Typically, the injectate includes 40 mg Triamcinolone, if the patient has a hemoglobin A1c < 8.0, and a mixture of 10-19cc of NS or sterile water and 10 cc Ropivacaine. In the poorly controlled diabetic patient with hemoglobin A1c > 8.0, there is the option to substitute steroid with injection of 30mg – 60 mg of Ketorolac, provided that renal function is preserved.
MANUAL THERAPY PROGRAM
Pre-Injection
Pre-injection measurements of ROM by the PT should be obtained to provide an objective baseline for the assessment of progress. Active range of motion (AROM) measurements are taken in sitting and passive are taken in supine, using standard techniques. At a minimum, seated AROM of the involved shoulder should consist of flexion / elevation, functional internal (IR) and external rotation (ER) with the arm at the patients’ side. Additional measurements should include IR and ER at 45 and 90 degrees of abduction (Table 2). These measurements should be taken prior to injection as well as during any follow up appointments.
Post-Injection
Additional details of the initial visit are provided in Table 2.
Supine Passive Range of Motion (PROM) measurements are the focus following the intervention on day one. Seated AROM measurements can be a challenge to acquire that early following the procedure.
Immediately after the brisement procedure (by their physician) the patient is seen in physical therapy and is placed in a supine position of comfort. Manual techniques on average begin within 10 minutes of the injection. To get a baseline assessment and develop a degree of patient comfort with mobility, the upper extremity is passively moved gently into flexion and then abduction, avoiding any pain or end point stretching. This is followed by the same for IR and ER, performed with the humerus supported by a towel in 45 degrees of abduction. The patient is to notify the therapist if the pain reaches a 4-5/10 level (on Verbal Numerical Rating Scale), as this will often be a stopping point for a technique to avoid aggravation.
Following the passive range of motion, soft tissue work is performed to reduce the reflexogenic tonic guarding of the rotator cuff and pectoral muscles. The most tonic of these muscles need to be addressed to improve ROM. The pectoralis minor, subscapularis, infraspinatus, and teres/latissimus complex are the most common muscle groups in the shoulder complex that develop increased tone and will often require attention during treatment. Interventions consist of scapular mobilization, soft tissue mobilization, joint articulation, and forms of stretching when tone is reduced such as a proprioceptive neuromuscular facilitation hold relax. For this reason, the patient is frequently treated in side-lying. This position is often preferred due to patient comfort and the ability of the clinician to address various angles of motion within the transverse plane while allowing for progressive ranges of abduction. As soft tissue restrictions in tone decrease, Grade 2 to 3 GH joint mobilizations laterally, inferiorly and less often, anteriorly or posteriorly may be performed. Additionally, the sternoclavicular, acromioclavicular, first and second rib, and thoracic regions should be evaluated and addressed with mobilizations if restrictions are present.
Home Exercise Program
As motion improves, home instructions are given to correct postural impairments and maintain mobility in supine and standing. Repetition number and hold times can vary depending on irritability levels, with longer holds in less irritated tissue. The focus on mobility is on frequency and repetition, not on intensity. The home exercise program (HEP) is initially performed in the department and cued for proper execution (Table 2). Adjustments can be made depending on patient characteristics.
Multiple components of education are provided after completion of the session including frequency of home practice and the expectation that discomfort should continue to diminish. Patients often experience fear-avoidance in the performance of the HEP and should be reminded that pain tolerance should be used to guide number of repetitions and duration of static hold, stepping up or down based on the experience of a 5/10 level Verbal Numerical Rating Scale. Best practices regarding posture and sleeping positions are discussed.
The initial HEP is to be performed one to three times daily depending on need. Those with greater restriction should be instructed for a higher frequency. Patients should return in one week for progression of mobility and muscle coordination training. The authors recommend one to three follow-up visits, as progress and needs dictate after each visit.
Those who experience improvements in range of motion generally retain or continue to experience progressive gains until the time of first PT follow-up. While the presence of more significant OA may inhibit gains in range of motion, improvement in pain often yields patient satisfaction in function. For this reason, changes in ROM should not be used as a primary predictor of function at the initial visit.
Physical Therapy Follow-Up
Follow-up therapy visits are used to track progress, advance exercises, and address muscle coordination, with the goal of improving shoulder mobility, strength, and function. The second PT visit begins with a warmup for 3 minutes, on the upper body ergometer or pulleys, followed by ROM measurements. If minimal gains are made, manual techniques and current home exercises are performed, and when able, progressed. If active ROM is improved and pain levels remain minimal, the individual may be started on AA/AROM (active assist or active ROM) in all planes while standing and possibly light motor recruitment and coordination exercises to scapular and shoulder muscles. Strength-focused activity can be initiated when functional ROM of approximately 80% is achieved. Manual interventions are at the discretion of the therapist as needed. Education and cues involving postural influences are reinforced.
The anticipated number of PT visits following brisement is generally less than four, and frequently only one to two. By discharge, patients should have a well-rounded shoulder routine. The general recommendations for mobility include 5 to 7 sessions at home per week in all planes of motion. The recommendation for strengthening exercises is a routine performed three times weekly of coordination and recruitment. Modifications are expected and education on exercise progression and regression principles should be discussed in detail.
ADVERSE EFFECTS
Standard of care injection precautions and risks including infection and bleeding should be discussed by the physician at the time of the procedure. Patients are instructed to report concerning symptoms at each point of medical contact.
There are no anticipated adverse effects related to therapeutic interventions beyond what would be expected during manual testing. Patient-provided feedback regarding pain tolerance during manual techniques and therapeutic exercises is important to avoid aggravation of tissue.
COORDINATION OF CARE
As this is a team-based approach, involving a temporal interaction with the patient, physician, and physical therapist, it is critical for the physical therapist to have space and time to work with the patient before and after the procedure. In traditional clinic-based settings this may pose a challenge. Success can be achieved with partly coinciding visit slots available on the physician and PT schedules labeled ‘TEAM’ visits to allot time for both parties to see the patient in the physician’s office. This may be done with greater ease when the PT practice is near or adjoining to the physician’s clinic. For example, advanced scheduling is crucial and is recommended to be done by office staff with access to both parties’ schedules. After physician consent for the procedure, initial PT assessment and measurements can be taken, followed by physician-performed US guided injections, then post-injection therapy by the PT. In this manner, both the therapist and physician have flexibility while the other is in the room working with the patient.
LIMITATIONS
There may be barriers to the successful execution of this collaborative treatment. First, the administering physician must have accessibility to and skill with MSK-US. To ensure precise placement of peri-neural anesthetic and additional injection under the joint capsule, the authors do not recommend proceeding without ultrasound guidance. Insurance coverage of and patient-belief towards PT could also influence feasibility of performance, and financial limitations should be addressed prior to scheduling. Generally, ultrasound guided GH injections are covered by majority of insurances in the USA. Lastly, patients may have medical contraindications to corticosteroids and NSAIDs, which would make them unsuitable candidates.
CONCLUSION
This clinical commentary presents a novel treatment strategy to address significant pain and functional impairment associated with GHOA. To the authors’ knowledge, this is the first publication describing the use of hydrodilatation of the GHJ to treat symptomatic GHOA. The approach includes a team-based strategy, combining the ultrasound-guided brisement procedure and nerve block with a structured physical therapy protocol.
This innovative application of a practiced procedure with physical therapy collaboration can improve range of motion, reduce pain, and enhance strength and function in GHOA. It is applicable to a wide patient population, with only few contraindications and does not require ‘down’ time.
Following publication of this clinical commentary, future research directions will include a proof-of-concept study analyzing both short- and long-term outcomes of the brisement hydrodilatation procedure for GHOA. Overall, this multidisciplinary approach offers a promising, minimally invasive option for patients with GHOA, potentially improving clinical outcomes and quality of life while minimizing the need for surgical intervention.
Disclosures
Adam Kimberly- is a paid lecturer with Optimal Dry Needling Solutions via IAOM
Jason A Genin – is a paid consultant for Hydrocision
Richard Kring- is a paid consultant for Cleveland Clinic international partners and personal clients. He received honoraria for lectures presented at state, national or international conferences and universities.
Leo Oliviera- receives teaching payments from Trice Medical, Lipogems, Bioventus
Corresponding Author
Jason A. Genin, DO
33100 Cleveland clinic blvd
Dept of Ortho
Avon OH 44011
Fax: +1 440-695-4189
Telephone: +1 216-339-9768
Email: GeninJ@ccf.org
_block_performed_at_the_spinoglenoid_notch_via_.png)
_block_performed_at_the_spinoglenoid_notch_via_.png)
