Introduction
Over the past decade, the incidence of anterior cruciate ligament injury and reconstruction (ACLR) procedures in young adults has increased.1–3 Gait asymmetries persist for six months to one year after ACLR and have been associated with the development of early onset radiographic osteoarthritis (OA).4–7 Athletes who developed radiographic knee OA five years after ACLR walked with underloading of the medial compartment of the tibiofemoral joint of the ACLR knee compared to those who did not develop radiographic knee OA early after reconstruction.4 Slower walking speed may be a potential predictor for future OA development.8–12 An association between slower walking and greater serum collagen type II cleavage concentration, a biomarker associated with greater cartilage breakdown in the knee, have been found in individuals six months after ACLR.6,9
In healthy individuals, faster walking speeds are associated with larger joint moments and angles in the sagittal plane.11,13 Faster walking may promote joint loading, thus could be used as a potential intervention to prevent early onset OA. Faster walking speeds are also associated with lower incidences of knee OA in mid- to older-aged adults.10 The relationship between walking speed and knee joint moments and angles during gait after ACLR is unknown.
There are no interventions that fully restore gait symmetry early after ACLR. Perturbation training, weighted vest training, and sled towing have been trialed in the past but have been unsuccessful in restoring gait symmetry.14–19 Strength, agility, plyometric, and secondary prevention training and perturbation training interventions have been shown to help mitigate asymmetry in women two years after ACLR, but not at earlier timepoints.16 Real-time visual biofeedback with verbal cues suggest that individuals may be able to modulate medial compartment tibiofemoral contact forces, thereby improving symmetry.20,21 These bio-inspired technologies are, however, costly and time-consuming to implement in a clinical setting. Understanding the association between walking speed and knee biomechanics in athletes after ACLR may inform a clinically feasible intervention based on simply altering walking speed.
The purpose of this study was to determine the relationship between self-selected walking speed and gait biomechanics in athletes after ACLR. This study investigated the relationship between walking speed and knee biomechanics in the ACLR knee, in the contralateral knee, and for the interlimb differences (ILD = ACLR – contralateral knee). The first hypothesis was that faster walking speed would be related to larger knee kinematics, kinetics, and joint contact forces in both the contralateral and ACLR knees. The secondary hypothesis was that less asymmetry would be observed in gait biomechanics for those who walked faster.
Methods
This study is a secondary analysis of prospectively collected data from a clinical trial (NCT01773317) approved by the University of Delaware institutional review board. Written informed consent was obtained from all participants and from parents/guardians for participants who were minors.
Seventy participants (21±8 years old) (Table 1) were included in this study.22 Participants were level I and II athletes (i.e. sports involving jumping, pivoting, and cutting),23,24 who participated in their sport at least 50 hours per year prior to injury, underwent primary, unilateral ACLR, and planned on returning to their pre-injury level of sport after surgery. Athletes were eligible for study enrollment 12 weeks after ACLR. Criteria were: minimal to no effusion,25 full and symmetrical knee range of motion, ≥80% quadriceps index ([ACLR knee maximal volitional contraction (MVIC) / contralateral knee MVIC] x 100), initiation of a running progression, and ability to hop pain-free on each leg. Participants were excluded if they had a previous history of ACLR or other significant lower extremity injury or surgery on either knee, concomitant grade III knee ligament injury, or osteochondral defect ≥ 1 cm2.22 All participants from the parent clinical trial whose biomechanical testing data were able to be modeled were included in the present study.
Motion Analysis and Variables of Interest
Motion capture and EMG (1080Hz) data were collected 24±8 weeks after ACLR. Commercial software (Visual3D; CMotion, Germantown, MD) was used to calculate kinematic and kinetic variables via inverse dynamics. Thirty-nine retroreflective markers were placed on both lower extremities, and kinematic data were captured at 120 Hz using an eight-camera motion analysis system (VICON, Oxford, UK). Kinetic data were recorded using an embedded force platform (Bertec Corporation, Columbus, OH) sampling at 1080 Hz. Five over-ground gait trials were collected at self-selected walking speeds maintained at ± 5%. Electromyography (EMG) was collected via electrodes placed bilaterally on the rectus femoris, medial and lateral vasti, medial and lateral hamstrings, and the medial and lateral gastrocnemii. We collected MVICs for each muscle group and data were normalized to each muscle’s MVIC. EMG data were high-pass filtered at 30 Hz using a 2nd order Butterworth filter and low-pass filtered at 6 Hz to create a linear envelope.
Variables of interest were external peak knee flexion (pKFM) and adduction moments (pKAM), peak knee flexion angle (pKFA), and knee flexion (KFE) and extension (KEE) excursion. KFE was defined as the change in knee flexion angle from initial contact to peak knee flexion. KEE was defined as the excursion between peak knee flexion angle and peak knee extension angle during the second half of stance. Joint moments were normalized by mass and height (kg×m). pKFA and pKFM were normalized so that positive values reflect greater knee flexion angles and external knee flexion moments, respectively.
Musculoskeletal Modeling
A validated, patient-specific EMG-driven model was used to calculate the primary variable of interest, medial tibiofemoral joint contact forces (pMCCF),26,27 which has been associated with early onset OA.4,5,28 The previously validated26,27 model uses a hill-type muscle fiber in series with an elastic tendon. EMG-derived forward dynamics estimations are used to create a knee flexion moment curve, which is then fit to the knee flexion moment curve generated through inverse dynamics calculated through motion capture and ground reaction forces. We then predicted each of the five trials per knee using muscle parameters and coefficients (pennation angle, fiber velocity, tendon strain, and muscle activation) from the other four trials. The three best-fitting predicted trials were selected by maximizing R² and minimizing root mean square error values. A frontal plane moment balance algorithm was used to calculate pMCCF, the modeling-derived variable of interest.22,26,27 pMCCF was normalized to body weight to allow comparison between individuals.
Statistics
Pearson’s correlation coefficients were computed for the variables of interest (contralateral knee, ACLR knee, and ILD) and walking speed. Linear regression was computed for all variables against walking speed to assess the linear relationship between each variable and walking speed. Alpha was set at 0.05 for all statistical analyses. Analyses were computed using SPSS version 25.0 (IBM Corporation, Armonk, NY).
Results
Contralateral Knee Mechanics
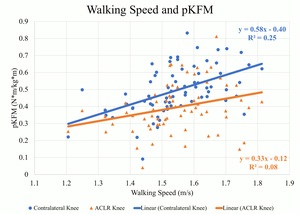
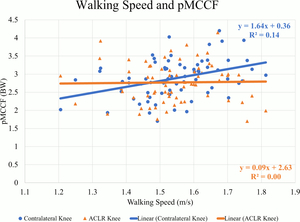
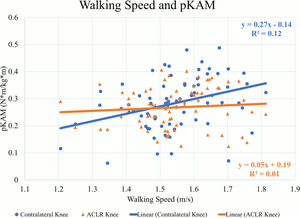
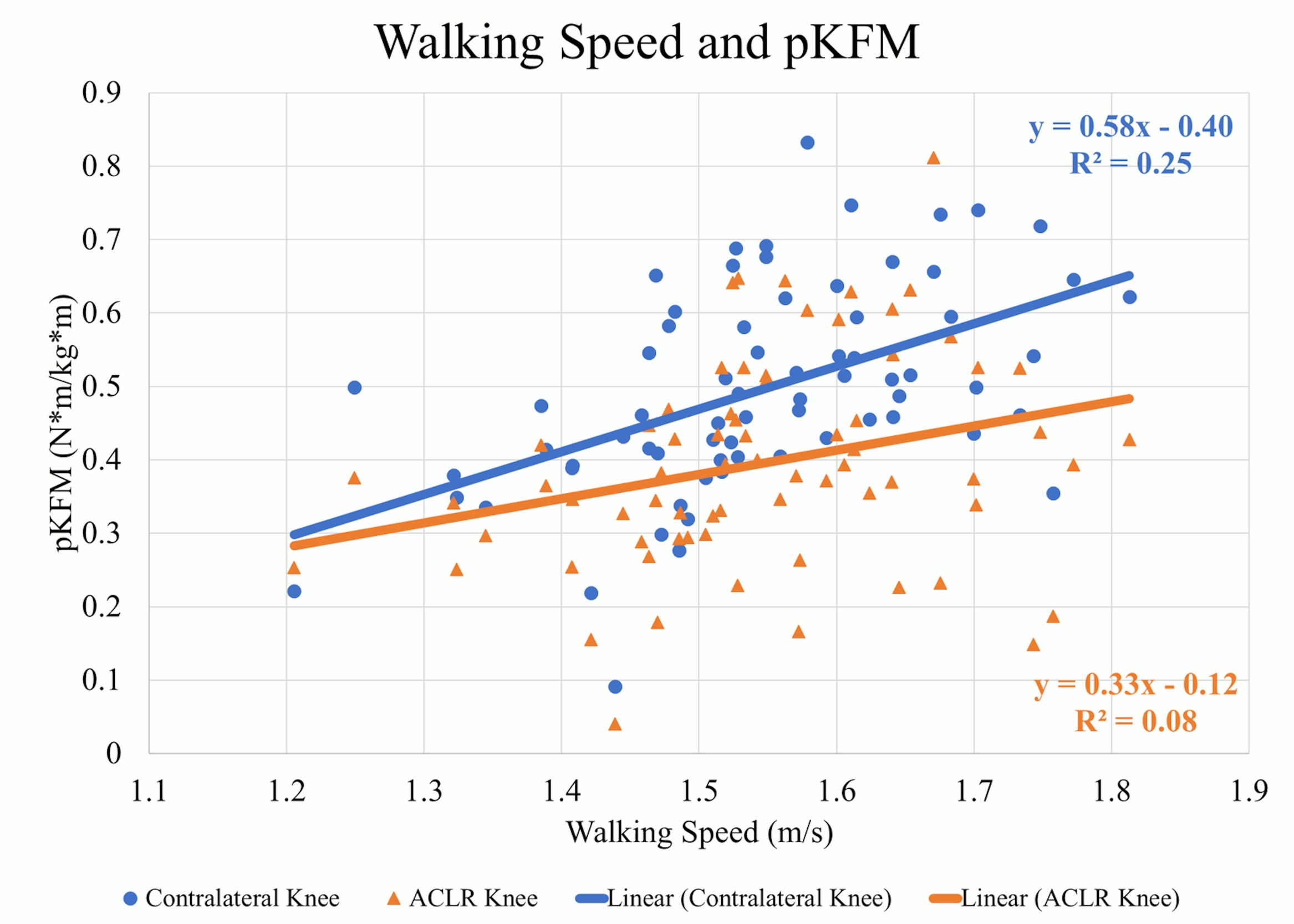
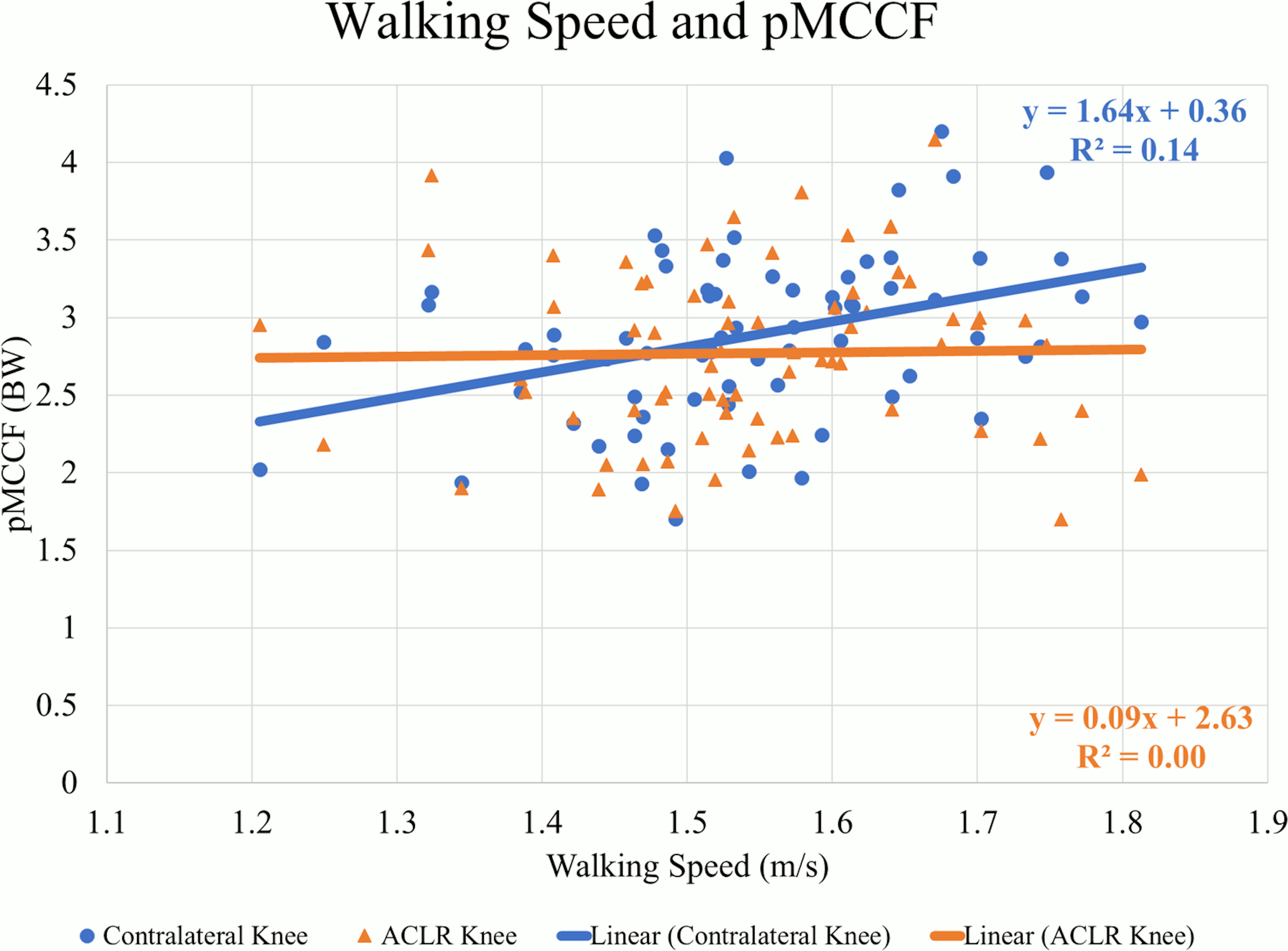
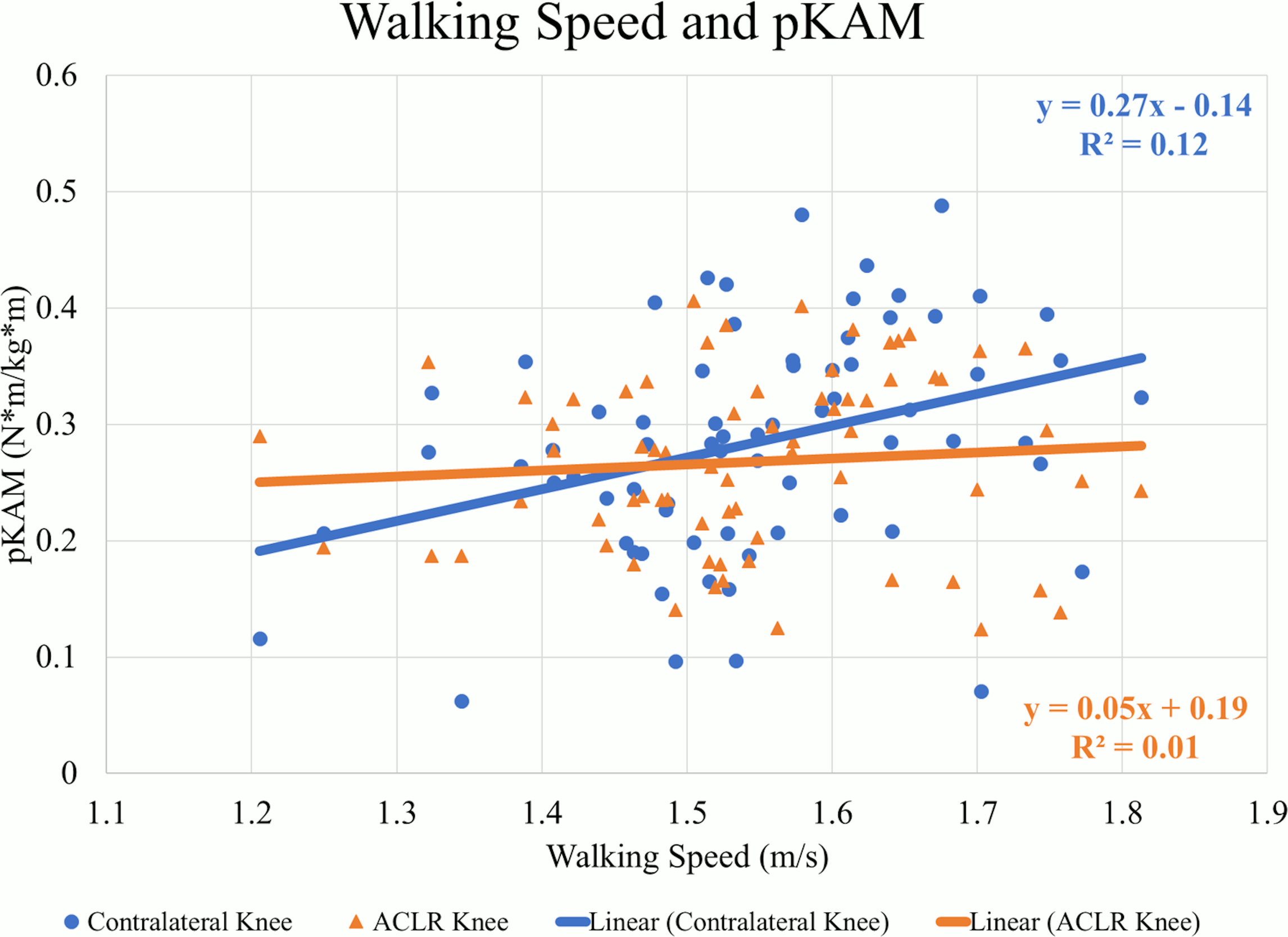
There were weak to moderate positive correlations between walking speed and mechanics indicating larger values occurred at faster speeds in the contralateral knee. At faster speeds, pKFM (Pearson’s r=.505, p<0.01) (Figure 1), pKFA (r=.449, p<0.01), KEE (r=.379, p<0.01), pMCCF (r=.377, p<0.01) (Figure 2, Table 2), pKAM (r=.346, p<0.01) (Figure 3), and KFE (r=.301, p=0.01) were greater in the contralateral knee.
ACLR Knee Mechanics
There were weak positive correlations observed between walking speed and pKFM (r=.280, p=0.02) (Figure 1, Table 3) and pKFA (r=.263, p=0.03) in the ACLR knee. However, there were no statistically significant correlations between walking speed and pMCCF (r=.020, p=0.87) (Figure 2), pKAM (r=.080, p=0.49) (Figure 3), KFE (r=.065, p=0.59) and KEE (r=.226, p=0.06) suggesting no strong relationship between walking speed and gait mechanics in the ACLR knee.
Interlimb Difference
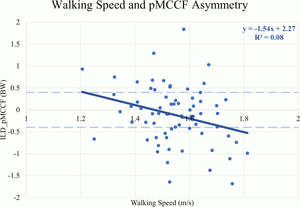
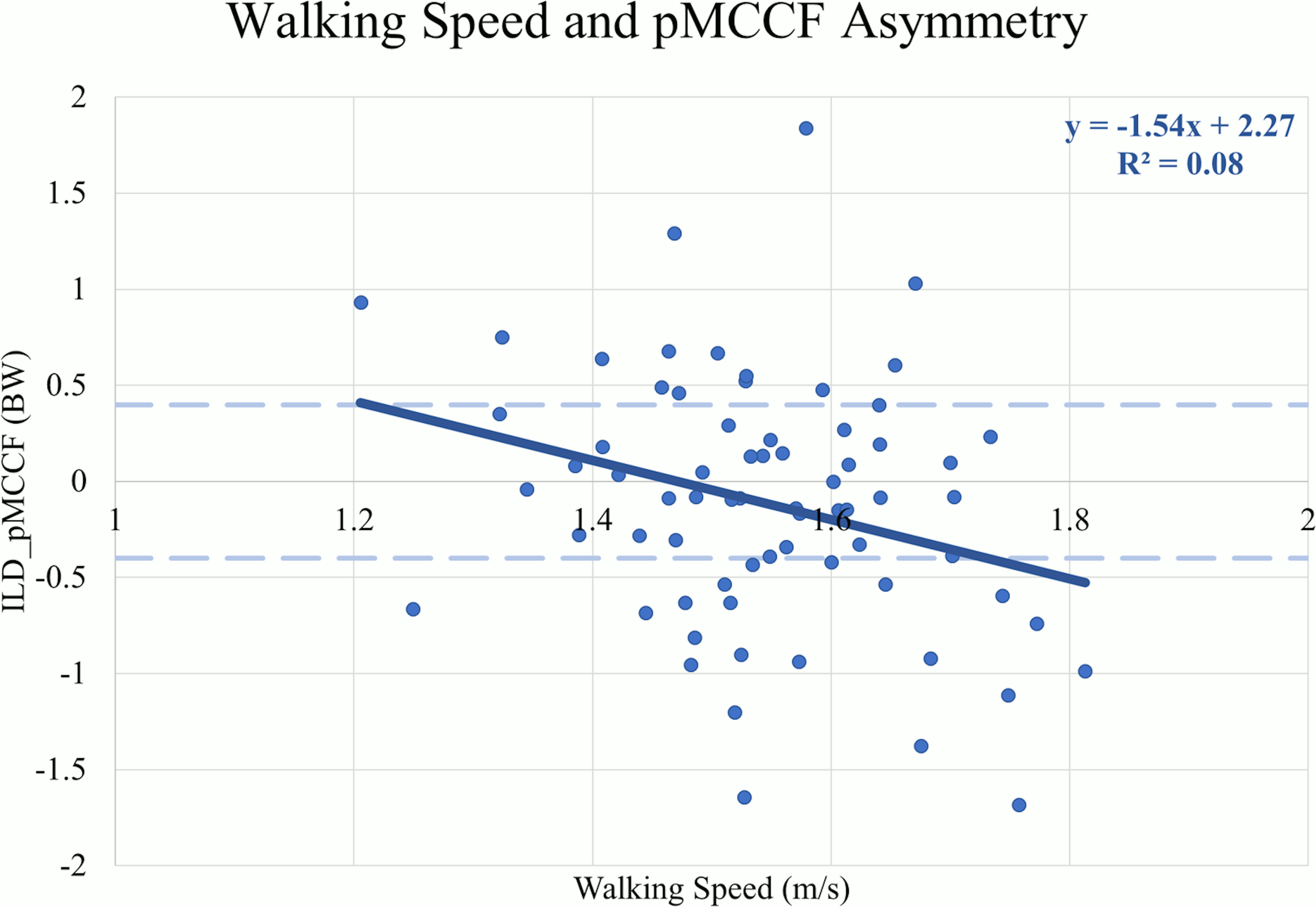
There were weak negative correlations between walking speed and ILDs for KFE (r=-.260, p=0.03) (Table 4), pKAM (r=-.323, p<0.01), pMCCF (r=-.286, p=0.02) (Figure 4), and pKFM (r=-.248, p=0.04) indicating that more asymmetry occurred at faster speeds. There were no correlations between walking speed and ILDs for pKFA (r=-.248, p=0.07) or KEE (r=-.172, p=0.15).
Discussion
This study explored the relationship between self-selected walking speeds and gait biomechanics in athletes after ACLR. The first hypothesis, that faster walkers would have greater knee moments, angles, and joint contact forces, was supported in the contralateral knee but only partially and weakly supported in the ACLR knee. Only pKFM and pKFA in the ACLR knee were weakly correlated to walking speed. The secondary hypothesis, that there would be less asymmetry at faster walking speeds, was not supported. Greater asymmetry was observed for pKFM and KFE at faster walking speeds, and beyond 1.5 m/s in pMCCF (trending toward underloading) and pKAM. The ACLR knee does not present with the same characteristics as the contralateral knee at faster walking speeds, suggesting that healthy responses to faster walking13 may not be observed in the ACLR knee.
Gait asymmetries persist six months after ACLR in this and other cohorts.5,16,29–31 Given that previous research suggests that faster walking leads to larger angles and moments, especially in the sagittal plane,13 the hypothesis was that there would be similar trends in both the ACLR and contralateral knees. The findings in this study, conversely indicate that those who walk faster six months after ACLR, compared to those who walk more slowly, actually have more gait asymmetry. These results indicate that walking speed is more strongly associated with contralateral knee biomechanics than ACLR knee biomechanics. The varying strength of the relationships between each limb and walking speed may explain the underlying resultant asymmetry (i.e., underloading of the involved knee) observed for those who walked faster.
For all sagittal plane variables (PKFA, PKFM, KFE, and KEE), asymmetries were present regardless of walking speed, and were exaggerated for those who walked at faster speeds. The line of best fit for the association between walking speed and the ILD in pMCCF, the primary variable of interest associated with OA development,4,5,28 and between walking speed and the ILD in pKAM both crossed zero at approximately 1.5 m/s. Individuals who walked faster tended to exhibit greater underloading in the ACLR knee relative to the contralateral knee (Figure 4), an association that was driven primarily by changes in the contralateral limb (Figure 2, Table 2). As illustrated in Figure 4, clinically meaningful pMCCF underloading, as determined by ILDs exceeding the meaningful interlimb difference threshold of 0.4 BW,4 was more prevalent at faster speeds than was pMCCF overloading. These relationships were similar for men and women, as shown in secondary exploratory analyses (Table 5). Therefore, an intervention that manipulates walking speed alone for asymmetric gait mechanics after ACLR may result in continued gait asymmetry, perhaps even increasing the asymmetry. Future research, however, must manipulate walking speed within individuals after ACLR to determine its effect on walking symmetry.
The mechanism underlying the prolonged gait asymmetry following full functional recovery after ACLR is unknown. One possible explanation is neuroplastic changes after injury and reconstruction.32 Sigward et al. suggests that individuals three months after ACLR exhibit a mismatch between their loading patterns and abilities – termed “learned nonuse” – during bilateral tasks. Sit-to-stand and static standing are activities that are typically performed without attention to loading, similar to gait. Individuals in the Sigward et al. study exhibited asymmetry without cuing during the tasks, but were able to load symmetrically (measured by vertical ground reaction force impulse) once real-time visual and verbal feedbacks were provided.33 Pizzolato et al. found real-time visual biofeedback with verbal cues during gait may have short-term effects on restoring gait symmetry including variables such as medial compartment tibiofemoral contact forces.20,21 These were short-term studies and visual feedback has not been shown to improve long-term learning or transfer to other environments. With repetition and targeted intervention, however, these changes may carry over into long-term gait symmetry. Given these findings, individuals early after ACLR may benefit from external feedback focusing on neuromuscular control in the ACLR knee in order to learn to load symmetrically during gait.32
Interventions such as split-belt treadmill training may allow clinicians to unilaterally target the ACLR knee by decoupling the belts so that one limb moves faster than the other. Roper et al. found that split-belt training has the potential to increase hip adduction moment impulse of the fast limb.34 Therefore, targeting the ACLR knee using gait-specific, unilateral neuromuscular retraining early after ACLR may be promising for improving symmetry of knee moments, angles, and joint contact forces. Future research is warranted.
While the cause and effect of walking speed and underloading is unclear, both slower walking9,12 and tibiofemoral underloading5,11,16,18,28 of the ACLR knee at six months after ACLR have been associated with early OA development. In the present study, faster walkers tended to walk with tibiofemoral underloading of their ACLR knee at six months following ACLR. In contrast, slower walkers had more symmetrical medial compartment loading. These unexpected associations, however, were driven almost exclusively by the contralateral knee rather than the ACLR knee. Individuals who walked faster did not load their ACLR knee less than individuals who walked more slowly (Table 3); they only underloaded their ACLR knee relative to the contralateral limb, which experienced higher loading at faster speeds (Table 2). Optimal tibiofemoral joint loading and walking speeds early after ACLR remain unknown. Further follow-up including musculoskeletal imaging is necessary to elucidate the relationship between joint loading, walking speed, and long-term OA development.
This study was an explorative secondary analysis, so the interventions discussed are speculative in nature and should not be taken as clinical practice recommendations. The present study is limited by its cross-sectional, between-subjects design; walking speed was not manipulated. Additionally, correlations were weak to moderate. This sample may not be representative of the general ACLR population due to the stringent inclusion criteria (level I and II athletes, participated in their sport at least 50 hours/year prior to injury, primary, unilateral ACLR, and planned to return to pre-injury level of sport after ACLR). Lastly, this sample does not include long-term radiographic evidence of OA development.
Conclusion
The results of this study indicate that contralateral knee mechanics and loading were more strongly correlated to walking speeds (i.e., greater angles, moments, and loading with faster walking) than ACLR knee mechanics and loading. This difference may explain the larger gait asymmetries observed in faster walkers. Further research is warranted to study targeted interventions to improve gait symmetry for individuals after ACLR. These results suggest that those who walk faster demonstrate greater interlimb asymmetry, rather than less asymmetry. Therefore, manipulating walking speed alone as an intervention for targeting aberrant gait mechanics may accentuate, rather than mitigate, walking asymmetries among individuals after ACLR. Further research is necessary to understand the long-term effect of different walking speeds on gait biomechanics within the same individual. Gait-specific, unilateral, neuromuscular training for the ACLR knee may be necessary to improve symmetry in postoperative rehabilitation.
The Institutional Review Board of the University of Delaware approved this study, which was registered on IRBNet (ID 225014-15). This is a secondary analysis of a clinical trial which was registered at clinicaltrials.gov (NCT01773317). This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR048212) and the National Institute of Child Health and Human Development (R37-HD037985, T32-HD007490, F30-HD096830). This study was funded in part by The Foundation for Physical Therapy Research Promotion of Doctoral Studies (PODS) Level I and II Scholarships (JJC), University of Delaware Doctoral Fellowship Award (JJC), Dissertation Fellowship Award (JJC), and Biomechanics and Movement Science (BIOMS) tuition scholarship (NI). JJC’s postdoctoral training is funded by an Advanced Geriatrics Fellowship from the Eastern Colorado Veterans Affairs Geriatric Research Education and Clinical Center. The authors certify that they have no financial disclosures or direct financial interest in the subject matter. The authors have no conflicts of interest to disclose.