INTRODUCTION
Adequate balance control is required for safe sport participation.1 Postural control is a complex task requiring continuous interaction and adaptation between sensory, motor and cognitive functions. The components of postural control have been conceptualized to include: (1) movement strategies, (2) control of dynamics, (3) sensory strategies, (4) cognitive contributions, (5) orientation in space, and (6) biomechanical elements.2 Postural impairments may result from deficits in one or more of these components.3
Sport-related concussion (SRC) is a traumatic brain injury induced by a biomechanical force.4 Impaired postural control is a common sign of SRC, presenting in up to 80% of athletes who suffer a SRC.5 Accordingly, postural control assessment is critical for SRC diagnosis and return-to-sport (RTS) decisions. The most commonly used clinical (i.e., non-instrumented) postural control assessment tools for SRC, the Balance Error Scoring System (BESS) and modified Balance Error Scoring System (m-BESS) are relatively inexpensive and easy to administer.4,6 These tools are based on the premise that SRC postural control impairments are the result of sensory deficits alone, which have been shown to resolve within three to five days following injury.7 However, more sophisticated laboratory assessments of postural control have demonstrated that SRC postural control impairments are also associated with motor and cognitive deficits that may persist beyond five days.2 Given that typical standing balance tests are unable to challenge cognitive and motor resources, additional tests are required to evaluate the potential postural control consequences of SRC.8–10
Given the inadequacies of common clinical tests and limited feasibility of using laboratory measures in clinical settings,11 it is plausible that an athlete may be cleared for RTS despite ongoing postural control impairments. This may increase their risk of future injury.12 In response to this problem, there is a need for comprehensive clinical methods to assess postural control following SRC.4,9,10 The primary objective of the current study was to describe the development of a comprehensive battery (the Functional Assessment of Balance in Concussion or FAB-C) that assesses the sensory, motor, and cognitive components of postural control that may be impacted by a SRC. Secondary objectives were to examine the feasibility of administration, and construct validity (i.e., correlations between individual tests included and the ability to identify differences in performance between uninjured active individuals and those who had recently RTS following SRC) of the battery.
METHODS
Development of the FAB-C Battery
The development of the FAB-C battery was guided by a recently proposed model of postural control assessment following SRC.13 This model proposed that a comprehensive assessment of postural control following a SRC should include clinical tests that challenge sensory strategies, control of dynamics, movement strategies, and cognitive contributions components of postural control under single-task, dual-task, and sport-specific testing paradigms.
To identify tests that challenge these multiple components of postural control under various testing paradigms for possible inclusion in the FAB-C battery, the research team initially searched the literature to identify existing clinical tests with established clinometric properties. This list was reduced by comparing existing clinical tests’ purposes (i.e., the evaluated component of postural control) and clinimetric properties. Findings from the steps aforementioned were used to develop an unrefined version of the FAB-C battery, which was further examined for feasibility and preliminary construct validity.
Testing the FAB-C Battery
Participants
A convenience sample of active (Cincinnati Sports Activity Scale level one or two),14 youth (13–17 years old) and young adult (18-24 years old) athletes who either recently returned to sport (RTS) following SRC or without a concussion were recruited from private physiotherapy clinics, sport organizations, through advertisements, social media, or word of mouth between December 2017 to May 2019. Participants with SRC must have been diagnosed with SRC as per the 5th International Consensus on Concussion in Sport4 and returned to sport (i.e., unrestricted return to practice, game, or competition) within the 60 days prior to testing. Uninjured participants were individuals who had not been diagnosed with SRC within the prior year. Participants were excluded if they were not active in recreational or competitive sport; reported a history of lower extremity injury that caused absence from recreational/sport activities greater than one week within the last three months; had inner ear or sinus infection over the week prior to testing, uncorrectable (i.e., neither with vision glasses nor contacts) vision dysfunction at time of testing, history of cognitive deficits such as concentration abnormalities, history of attention deficit hyperactivity disorder; or were non-English speakers. Ethics approval (No: Pro00077091) was acquired from the University of Alberta Health Research Ethics Board, and informed consent and/or assent was obtained from all participants prior to testing as appropriate
Procedures
Data were collected at either a university lab or a private physiotherapy clinic. On the day of testing, participants completed study questionnaires that gathered demographic and medical history information. Participants were familiarized with the FAB-C testing protocol and performed a warm-up (i.e., 1 minute of sidestepping, one minute of jogging backward, and three minutes of jogging forward)15 prior to data collection. The lead investigator, who is a physical therapist with seven years of experience in SRC management, scored the participants’ performance as they completed three trials of the FAB-C battery. Short rest breaks (i.e., one to two minutes) were provided between trials as needed. The lead investigator also recorded all feasibility outcomes of interest.
Outcomes
A questionnaire adapted from the Sports Concussion Assessment Tool–5th edition (SCAT5)16 was used to collect information on participants’ sex, age, primary sport and medical history (i.e., history of previous concussions, current medications, number of days since injury, number of days since RTS, the health care provider who made a diagnosis of SRC, and the health care provider who made a RTS decision). Feasibility outcomes included the number of participants who completed the entire assessment, potential for adverse events (i.e., falls, near-miss falls, injury, or increased symptoms), and burden (i.e., the cost of required equipment and time required to complete the assessment). FAB-C scores included the individual scores from the tests that made up the battery.
Analysis
Descriptive statistics [mean (standard deviation), median (range) or proportion as appropriate] were used to summarize all FAB-C clinical test outcomes. To evaluate the feasibility of the FAB-C, the percentage of participants who completed the entire FAB-C battery, the percentage of participants who demonstrated adverse events during and/or after testing, average time (in minutes) required to administer the FAB-C battery, and cost (in Canadian Dollars) of equipment required were calculated. Data from participants who completed the entire testing battery were included in the analysis. To evaluate the construct validity of the FAB-C, a multitrait Spearman’s correlation coefficient matrix was used to examine correlation patterns between clinical tests included in the FAB-C battery to identify whether they assessed similar or unique components of postural control.17 The non-parametric Spearman’s correlation was used given the small sample size in this study. If the Spearman’s correlation between two clinical tests was 0.7 or higher, it was assumed that one of them could be replaced with another clinical test based on tests’ purposes and clinimetric properties.18 An α level of 0.001 was chosen to judge significance to account for multiple comparisons. Differences in performance on the FAB-C battery between uninjured participants and participants who had recently RTS following SRC were calculated and reported using basic descriptive statistics [mean (standard deviation), median (range) or proportion as appropriate]. All analyses were performed using IBM SPSS 25 for Windows (Armonk, New York).
RESULTS
Development of the FAB-C Battery
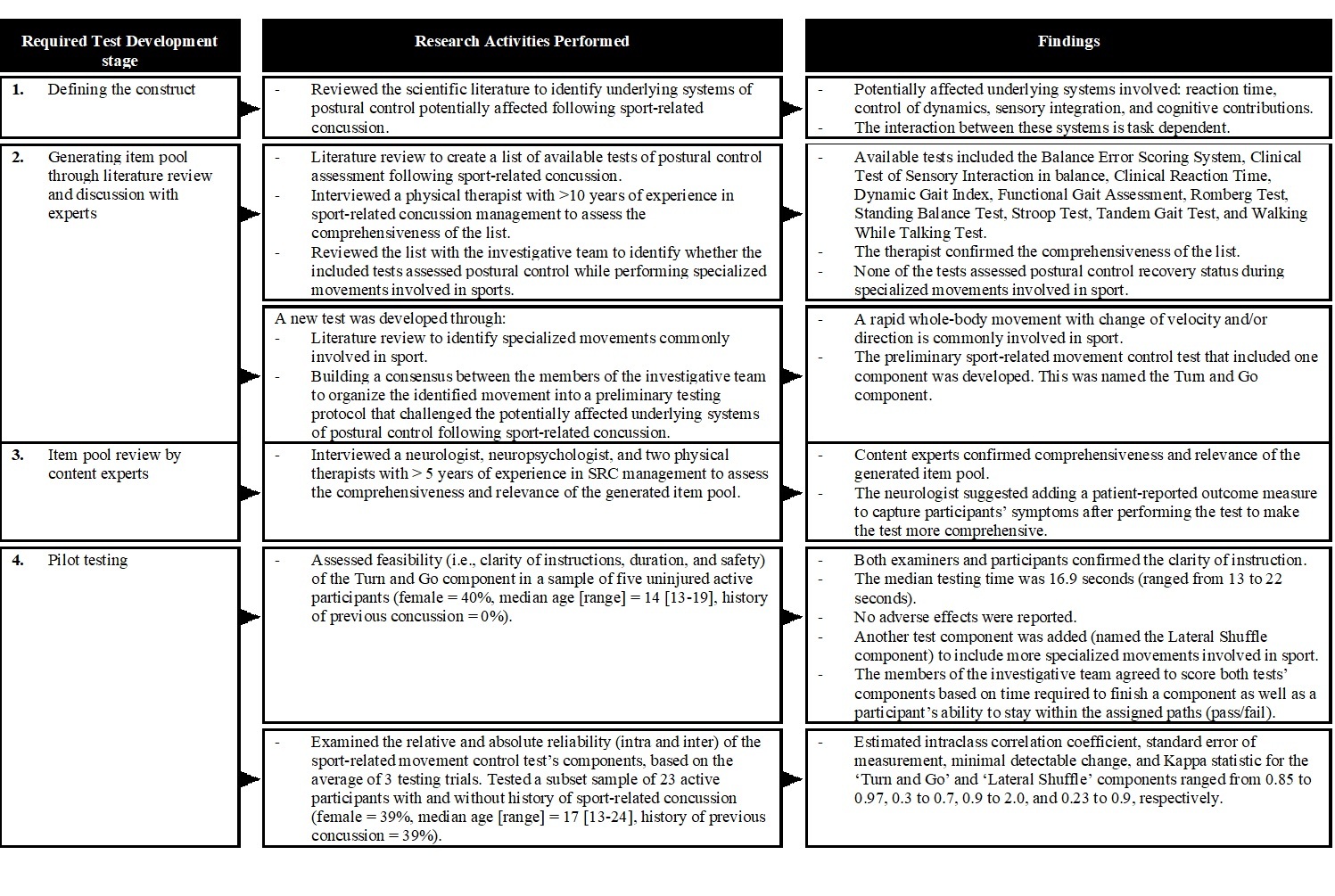
The literature search identified 13 clinical tests that were cited as appropriate in individuals SRC and were potential tests for inclusion in the FAB-C battery. Appendix 1 shows the tests with their purposes and established clinometric properties as well as the decisions used to include or omit each test in the FAB-C. This list was reduced to seven tests taking into consideration a tests’ purpose(s) and clinometric properties. These seven included the Balance Error Scoring System, Tandem Gait Test, and Clinical Reaction Time Tests in both single and dual-task (concurrent cognitive task) testing conditions. As no sport-specific testing paradigms were identified, the research team developed a Sport-Related Movement Control Test using the scale development framework of Johnson and Morgan (Figure 1).19 Finally, the research team incorporated a symptom checklist (The Post-Concussion Symptom Inventory)20 to ensure the FAB-C was comprehensive. A full description of these tests follows.
The Balance Error Scoring System. This test is commonly used to measure the sensory strategies component of postural control following SRC.4 The test involves the completion of three 20-second stance trials (i.e., double leg, single leg, tandem stance) on firm and foam surfaces. These are scored based on the number of errors committed (i.e., total score ranges from 0 – 60). For the dual-task condition, participants were asked to subtract by seven from a randomly assigned number while performing the test.21 The research team scored both the single- and dual-task Balance Error Scoring System by averaging the number of errors a participant commits from three testing trials to obtain the most reliable scores.22
The Tandem Gait Test. The test is currently an accepted measure of the control of dynamics component of postural control following SRC,4 and involves walking in a forward direction as fast and accurately as possible down and back along a 38mm-wide three-meter line using an alternate foot heel-to-toe gait. During the test, the administrator records the time (in seconds) required for participants to complete the test, as well as the participants’ ability to complete the test (i.e., pass/fail). For the dual-task condition, participants were asked to spell-out a five-letter word backward while performing the test.21 To obtain the most reliable scores for both the single- and dual-task Tandem Gait Test, the research team scored the time assessment by averaging time across three testing trials, and the pass/fail assessment based on a participant’s performance in trial three.22
The Clinical Reaction Time Test. The participants were asked to catch a numbered rod as quickly as possible. Drop distance is converted to speed. For the dual-task condition, participants were asked to verbally spell a five-letter word backward while waiting for the testing apparatus to fall.23 The research team scored both the single- and dual-task Clinical Reaction Time Test by averaging reaction time (in milliseconds) across three testing trials to obtain the most reliable scores.22
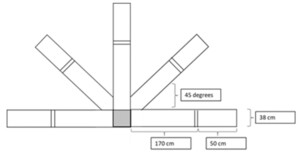
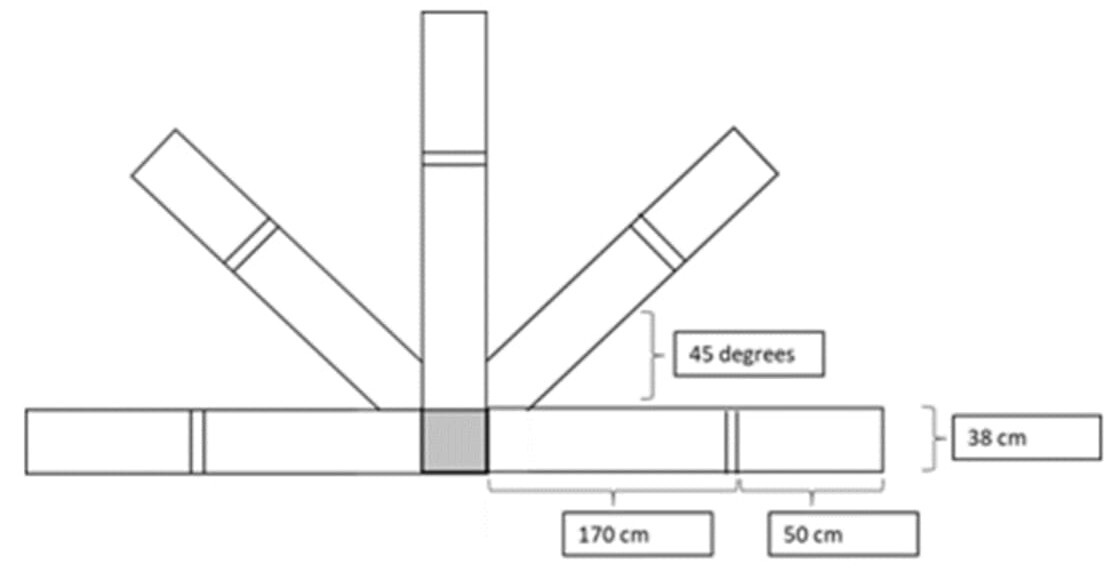
Sport-Related Movement Control Test. The test includes both a ‘Turn and Go’ (i.e., a sport-related movement control measure which involves forward running with repeated turning in five different directions within a limited base-of-support), and ‘Lateral Shuffle’ (i.e., a sport-related movement control measure which involves side-shuffle and backward running in five different directions within a limited base-of-support) components (Figure 2). Both tests’ components are scored based on time (in seconds) required for participants to complete the components (i.e., running in five directions), as well as the participant’s ability to complete the components (i.e., pass/fail). The research team scored the time assessment by averaging time across three testing trials, and the pass/fail assessment based on a participant’s ability to pass all three testing trials to obtain reliable scores (Figure 2).
The Post-Concussion Symptom Inventory. The Post-Concussion Symptom Inventory Self-assessment (ages 13 – 18) was used to document participants’ symptoms before and after testing. This version of the Post-Concussion Symptom Inventory has been validated for use with individuals following SRC, with acceptable test-retest reliability (intraclass coefficients [ICC] = 0.65–0.89).20
Testing the FAB-C Battery
Participant Characteristics
Uninjured (Table 1): Of 59 individuals who expressed interest in study participation, seven did not meet the inclusion criteria (history of concussion within the year prior to testing), three declined to participate (time constraints), and nine did not respond to communications, leaving a sample of 40 participants. The majority (70%) of uninjured participants reported hockey, basketball, ringette, or soccer as their primary sport and two (5%) participants reported current use of antibiotics for ongoing dermatological conditions.
Previous SRC with RTS in past 60 days (Table 1): Of nine individuals who expressed interest in participating in the study, one did not meet the inclusion criteria (history of lower extremity injury that caused absence from recreational/sport activities greater than one week within the last three months), and one declined to participate (time constraints), leaving a sample of seven participants. The majority (85%) of recently concussed participants reported football and hockey as their primary sport. Four (57%) participants were initially diagnosed with SRC by a physician, and three (43%) by an athletic therapist. Three (42%) and four (58%) participants RTS based on clearance from a physician and a physical therapist, respectively. The most frequently reported criteria for making RTS decision included symptoms resolution (43%), individual ability to perform physical tasks while being symptom-free (28.5%), or both (28.5%).
Feasibility
All (100%) uninjured participants completed the entire assessment on the FAB-C (see Table 2). No participants demonstrated adverse events during and/or after administering the FAB-C battery. The median total time needed to administer the FAB-C battery was 49 minutes (ranging from 44 to 60 minutes). The cost of equipment required to administer the FAB-C battery was ~$100 Canadian Dollars.
Construct Validity
Correlations between clinical tests included in the FAB-C battery ranged from -0.33 to 0.84 (see Table 3). Non-significant correlations between the Balance Error Scoring System, Tandem Gait Test, Clinical Reaction Time Test, and Sport-Related Movement Control Test were observed. Despite the high correlation between the Turn and Go and Lateral Shuffle components of the sport-related movement control test (r = 0.84, p < 0.001), the research team decided to keep both in the FAB-C battery given that each component involves a different set of movements required for sports participation (i.e. forward running with repeated turning versus backward running and side shuffle). The research team, therefore, did not remove any clinical tests from the FAB-C battery. Appendix 2 presents the final FAB-C battery inclusive of scoring, examiner, and patient instructions.
All (100%) uninjured participants and six (86%) participants who had recently RTS following SRC completed the entire FAB-C testing. One (14%) recently concussed participant withdrew after data collection due to the reproduction of SRC symptoms including headache, dizziness, and reported sadness. The percentage of uninjured participants who passed the single-task Tandem Gait Test, dual-task Tandem Gait Test, Turn and Go test, and Lateral Shuffle Test were 67%, 82%, 60%, and 52%, respectively; compared to 50%, 66%, 0%, and 17% of participants who had recently RTS following SRC, respectively (Table 2).
DISCUSSION
This paper introduces the FAB-C battery aimed at assessing different components of postural control relevant to SRC. The battery consists of seven performance-based clinical tests and a symptom checklist intended to be used in combination (and not in isolation) to determine a patient’s postural control assessment. The battery appears safe, feasible and inexpensive (i.e., its cost is comparable to the cost of the Balance Error Scoring System, which is commonly used for postural control assessment in SRC).6 However, the total time required to administer the battery is lengthy (44 – 60 minutes).
Although the FAB-C battery requires a considerable administration time, a comprehensive battery of tests that includes evaluation of various components of postural control that may be affected by SRC is needed and may help professionals to identify areas of ongoing dysfunction.4 In addition, specific components of postural control may benefit from targeted rehabilitation and may also identify additional areas that may increase the risk of subsequent musculoskeletal injuries and concussion.4 Moreover, the time required to administer the battery is comparable to that required to administer a comprehensive assessment of motor skills in individuals with SRC (e.g., Bruininks-Oseretsky Test of Motor Proficiency Second Edition) yet with a lower associated cost.50 To reduce the time of administration, future studies should examine the clinical utility of using total versus subdomain scoring of the FAB-C battery.
Data analysis showed limited correlations between individual tests included in the FAB-C battery. This indicates that each test assesses a different component of postural control and the tests should not be used interchangeably or in isolation when examining a patient’s postural control. The analysis also showed that all uninjured participants were able to complete the entire FAB-C battery; and passed the single-task Tandem Gait, dual-task Tandem Gait, Turn and Go, and Lateral Shuffle tests whereas only a proportion of recently RTS participants (Table 2). This observation provides preliminary evidence of the construct validity of the FAB-C battery to identify postural control impairments in youth and young adults who had recently RTS following SRC. This observation also supports previous studies suggesting that some athletes with SRC may RTS with residual postural control deficits.12,24
Future studies examining the proposed FAB-C battery in samples of individuals diagnosed with SRC of varying age, sex, gender, and sporting history are required before widespread use in clinical and clinical research settings. At this point, there is a need for studies examining the effect of different sources of variance (e.g., age, sex, and history of SRC) within individual clinical tests included in the FAB-C battery. Findings from these studies inform subsequent studies evaluating the clinimetric properties (i.e., validity and reliability) of the FAB-C battery. If the FAB-C battery is valid, reliable, and can differentiate which components of postural control are affected by a concussion, it could be used to inform the design and evaluation of rehabilitation strategies (i.e., selection of exercises that target affected components). Future studies may also examine the clinical utility capturing the accuracy of cognitive responses included in the battery.
Strengths and limitations
To the research team knowledge, the current study presents the first clinical assessment of postural control that aims at differentiating between various potentially affected components of postural control following SRC. This study, however, has limitations. Specifically, the research team recruited uninjured athletes who had not experienced a SRC over the year prior to testing, and analyzed data from only participants who completed the entire assessment, which introduced potential selection bias.25 The clinical tests used under the dual-task and sport-specific domains of the FAB-C battery have not been previously validated, which introduced potential measurement bias.25 Finally, the generalizability of the our findings from the current study is limited as the majority of the recruited sample involved uninjured athletes (85%), male athletes (63%), and athletes who played hockey, ringette, basketball, or soccer (60%).
Conclusion
The proposed FAB-C battery aims at differentiating between the potentially affected components of postural control following SRC. The results of this study indicate that the battery appears safe, feasible, inexpensive, and demonstrated preliminary construct validity to identify postural control impairments in youth and young adults who had recently RTS following SRC. Further studies evaluating the clinimetric properties and clinical utility of the FAB-C battery are required before adoption for widespread use in clinical settings.
COMPETING INTERESTS
The authors acknowledge that they have no conflicts of interest that are directly relevant to the contents of this research study.