Introduction
It is estimated that up to 250,000 anterior cruciate ligament (ACL) injuries are sustained each year.1 Standard of care in the United States after complete ACL rupture is surgical ACL reconstruction (ACLR), with over 125,000 performed annually.2 Individuals after ACLR typically demonstrate persistent neuromuscular deficits at the knee joint including, but not limited to muscular weakness,3,4 reduced proprioception,5,6 and altered walking7 and landing patterns8 characterized by altered knee biomechanics. Post-operatively it is recommended to restore thigh muscle performance.9,10 However, individuals after ACLR also appear to demonstrate persistent proximal neuromuscular impairments.11 These include altered hip abductor activation,12 hip extensor strength,13 reduced core motor control,14,15 and trunk extension endurance deficits.16
Neuromuscular fatigue can be described as the failure of a muscle to maintain a required or expected force output.17 Additionally, it has been suggested that experimental fatigue of a muscle may affect that muscle’s contribution to a task.18 The effects of experimentally-induced proximal neuromuscular fatigue on lower extremity biomechanics have been explored in a growing body of literature, predominantly in healthy and able-bodied individuals.18–23 Overall, it appears that when targeting specific muscle groups with a fatigue protocol, alterations in kinematics or kinetics are mainly observed in the primary plane of movement of those muscle groups.18–23 These altered movement patterns have also been observed in joints distal to the fatigued muscle group.19,21–23 In a previous related study, an isometric fatigue protocol targeting the hip extensors led to an increase in gluteus maximus muscle activation with preservation of joint kinematics during bilateral jump-landings in healthy individuals.18 Fatigue of muscle groups proximal to the knee region overall appears to impact instrumented assessments during clinical tasks in healthy populations, but has not been tested after ACLR.
Imposing neuromuscular fatigue on proximal muscle groups may interact with persisting post-surgical deficits after ACLR to exacerbate or reveal aberrations in movement reflecting further compromise in neuromuscular control.24 As lower limb neuromuscular control reflects a multi-joint coordinated strategy, analysis of total support moment (TSM) may provide novel insights into whole-limb movement strategies beyond that of single-joint parameters. The TSM is a summed moment from the extensor synergy of the hip, knee and ankle that represents the anti-gravity support of the center of mass of the body.25 At the instance of peak TSM, the sagittal plane moments of the hip, knee, and ankle are assessed to calculate the contribution of each individual joint to the TSM. The TSM has previously been used to characterize differences in neuromuscular strategy during single-limb landing after ACLR compared to controls.26
Most previous studies involving fatigue after ACLR have utilized peripheral fatiguing tasks such as squatting and jumping to functional failure points.24,27–31 However, these fatiguing tasks have not directly targeted proximal neuromuscular control. With relevance to the current study, previous electromyography work has validated the ability of the isometric Biering-Sorensen test procedure to induce fatigue to the gluteus maximus, the body’s primary hip extensor muscle.32 While the Biering-Sorensen test has been used to assess the effects of a hip extensor fatigue protocol on landing mechanics in healthy individuals,18 the effects after ACLR are unknown.
Therefore, the purpose of this study was to examine the effects of a proximal extensor musculature fatigue protocol on drop vertical jump landing biomechanics of individuals with a history of anterior cruciate ligament reconstruction using both single-joint parameters and total support moment analysis. The DVJ was utilized as the landing task of interest as it has been prospectively linked to secondary ACL injury risk,33 and has been evaluated before and after Biering-Sorensen testing in healthy individuals.18 It was hypothesized that the injured limb would differentially demonstrate greater peak lower limb flexion single-joint parameters after the fatigue protocol. Instances of increased hip contributions to movements have been seen in individuals with a history of ACLR during landing34 and squatting.35 Based on this data, it was hypothesized that the within-limb TSM joint contribution profile would shift distally to reflect a decreased hip contribution, with the injured limb redistributing less to the knee than the uninjured limb.
Materials and Methods
Design
This study was a single group, pretest-posttest quasi-experimental study. Participants were recruited with electronic and paper flyers. Individuals presented to the laboratory and provided written informed consent approved by the university’s Institutional Review Board prior to testing. The same examiners conducted all testing.
Participants
A convenience sample of nineteen participants between the ages of 18-40 years old with a history of unilateral ACLR at least one year prior was recruited for this study. To be included, participants needed to have completed formal rehabilitation and been fully cleared by a physician for return to sport or previous level of activity. Participants were excluded if they had any spinal or lower extremity pain or injury within the prior six months. Participants completed the Tegner Activity Scale and the International Knee Documentation Committee Scale (IKDC)36 prior to motion capture.
Motion Capture
Video data were collected using an 8-camera motion capture system (VICON, Centennial, CO, USA, 150 Hz), and force data using two force plates (BERTEC Corp., Worthington, OH, USA, 1500 Hz). Individuals had 47 reflective markers placed over their bilateral lower extremities and pelvis. Marker locations included: L5-S1 interspinous space, iliac crests, anterior superior iliac crests, greater trochanters, medial and lateral femoral condyles, medial and lateral tibial plateaus, medial and lateral malleoli, first and fifth metatarsal heads, distal feet, and proximal, distal, and lateral heels. Rigid marker clusters were placed over the thigh and shank segments. Static standing and dynamic calibration trials were captured.
Drop Vertical Jump
The DVJ was performed as previously described.37 Briefly, participants stood atop a 30 cm box. They were instructed to fall off the box, land with one foot on each force plate and immediately perform a maximal effort counter-movement jump reaching with both hands for an overhead target. For a trial to be successful the following criteria were required: participants dropped off the box without jumping, landed with each foot on a force plate, and successfully reached overhead during the jump. After successfully completing four trials, participants completed the fatigue protocol, with special care taken to prevent reflective marker placement alteration. Once the fatigue protocol was completed, participants immediately performed four additional successful drop vertical jump trials. All trials were used for analysis.
Fatigue Protocol
Participants completed three repeated efforts of a modified Biering-Sorensen extension position,15,29 held until failure, modified in that three repeated efforts were performed rather than one. Individuals had their lower extremities fastened to a plinth with two belts. Using upper extremity support, they initially positioned their torso horizontally off the plinth. To begin each effort, they were instructed to hold their torso parallel to the ground, to cross their arms, and to maintain that position for as long as possible (Figure 1). Participants were provided verbal encouragement throughout the three efforts. For each effort, one verbal warning was given when positional failure was observed. The second positional failure resulted in termination of that effort. Between efforts, individuals received a 15 second rest break. After completion of the final effort, the participants were immediately unfastened from the plinth to perform the post-fatigue DVJs. The time to termination for each effort was recorded, as was the time between the end of the final effort and the start of the post-fatigue DVJ procedures.
Biomechanical Data
Vicon Nexus was used for labelling and gap-filling of marker trajectories for the entire contact phase of the task, defined as the time in which a threshold of 20 Newtons of force was on the force plate. Trials were then exported and post-processed using Visual 3D (C-motion, Bethesda, MD, USA) and custom Labview code (National Instruments, Austin, TX, USA). Data were filtered using a low-pass Butterworth filter with a cutoff of 12 Hz. Joint angle and moment data were derived using X-Y-Z Cardan rotation sequences and traditional inverse dynamics procedures, respectively. Joint moments were normalized to body height (meters) and mass (kilograms) and reported as internal moments. Vertical ground reaction force (vGRF) was extracted and expressed normalized to body weight. For the single-joint parameters, the variables of interest were the sagittal plane peak joint angles and moments of the hip, knee and ankle, as well as peak vertical ground reaction force. For the TSM analysis, joint moment data were time normalized to ground contact, and were compared and summed at the instance of peak TSM.25
Statistical Analysis
Statistical analysis was performed using SPSS version 25 (IBM Corp, Armonk, NY, USA). Descriptive statistics were calculated on baseline parameters. Two-factor (condition-by-limb) analyses of variance with Bonferroni correction were performed to assess for potential interactions, main effects and simple effects for peak ground reaction force, peak joint moments and angles, and joint moments at the instance of peak total support. An alpha level of 0.05 was used. Post-hoc Pearson correlations were used to examine the relationship between change in percent contribution at the knee and hip to activity level (measured by Tegner), IKDC score, and time since surgery.
Results
Descriptive data for all participants are presented in Table 1. The average participant was a young adult female with a normal body mass index, approximately five years removed from unilateral ACLR with an IKDC score slightly below age matched healthy individuals36 and a Tegner score indicating recreational or competitive sport participation.
All participants completed all study procedures. In regards to the modified Biering-Sorensen fatigue protocol, an average reduction in time to positional failure of approximately 50 seconds (-59%) was observed between the first and third consecutive efforts. On average, participants transitioned to the post-protocol DVJ testing in less than 25 seconds after completing the third and final modified Biering-Sorensen effort (Table 2).
The single-joint results are presented in Table 3. No interactions or main effects of condition were observed. Two main effects of limb were seen. The injured limb demonstrated less peak ankle dorsiflexion angle (2°, p=0.028), as well as less peak vGRF (13%, p=0.013).
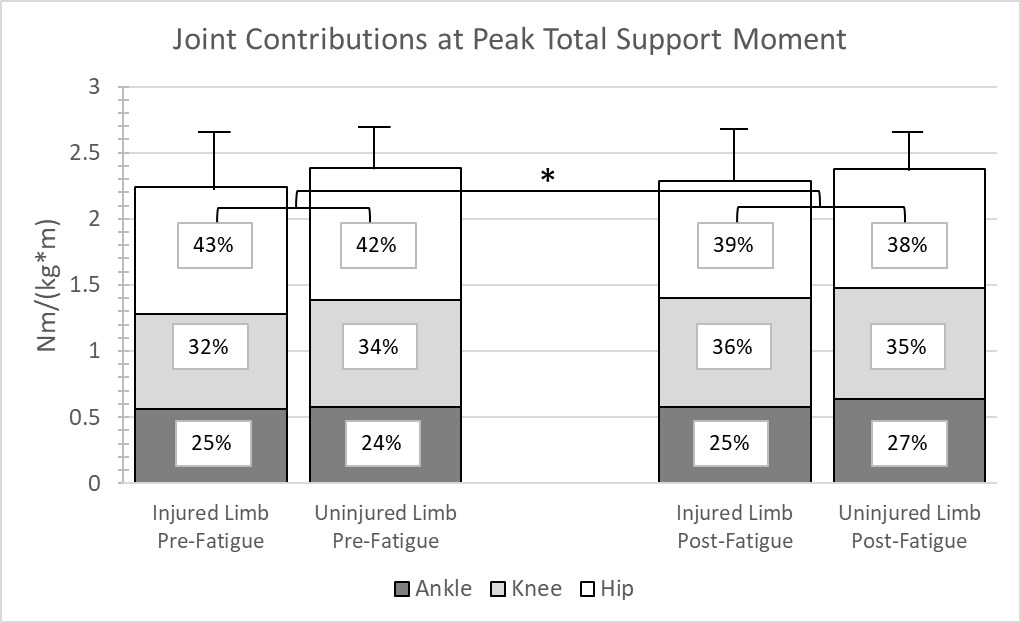
The joint contributions and peak TSM data are presented in Figure 2. No interactions or main effects of limb were observed. However, a single main effect of condition at peak TSM was seen for the hip contribution. After the protocol, at peak TSM, the hips showed a 4% decreased contribution to TSM (p=0.035).
Post-hoc analysis revealed a significant correlation (r = 0.527, p =0.02) between percent change in knee contribution post-protocol and time since surgery. The relationship was such that as time since surgery increased, individuals had a higher percentage contribution at the knee post-protocol. Otherwise, no other significant correlations were noted (p >0.05).
Discussion
The overall purpose of this study was to examine if a proximal extensor fatigue protocol elicited differential alterations in sagittal plane lower extremity kinematics and kinetics during a DVJ in individuals with a history of ACLR. Neither the single-joint nor whole-limb results directly supported the differential effect hypothesis, as no interactions were observed. When considering single-joint parameters, the participants loaded the injured limb less, and dorsiflexed less at maximum as well, which is consistent with findings in previous literature. When considering the whole-limb TSM analysis, the proximal extensor fatigue protocol generally elicited a decrease in the hip joint contribution to anti-gravity support, which provides supporting evidence that the fatigue protocol affected the targeted muscles.
Of particular relevance to the current study are the findings of Hollman et al.18 In a comparable study, healthy women performed a bilateral jump-landing task and isometric hip dynamometry before and after a modified Biering-Sorensen fatigue protocol. Hollman and colleagues measured hip and knee kinematics and gluteus maximus muscle activations, and found no change in peak hip and knee angles after the modified Biering-Sorensen fatigue protocol. However, peak isometric hip extension force was reduced and gluteus maximus electromyographic activation was increased after the fatigue protocol. The authors suggested that the kinematics may have stayed consistent due to increased neural drive to the gluteus maximus. In the current study, electromyographic data were not collected, and there there was no change in single-joint kinematics or kinetics supporting the results of Hollman and colleagues18 despite the history of unilateral ACLR. In regards to whole-limb neuromuscular strategy, the proximal extensor fatigue protocol resulted in a reduction in hip extensor contribution to peak TSM. Both the increased gluteal activations seen in the Hollman study and the altered TSM seen in this study may be considered evidence of compensatory motor solutions used to preserve requisite task-level kinematics.
Two discrete parameters were suggestive of altered function in the ACLR limb, peak vGRF and ankle dorsiflexion angle. In regards to decreased vGRF during landings, a recent systematic review with meta-analysis identified that 9/10 included studies showed reduced peak vGRF in adolescents with a history of ACLR.38 This study’s pre-protocol data add to the robust literature identifying loading asymmetry in individuals after ACLR. The lack of change in peak vGRF post-protocol suggests that proximal extensor fatigue does not strongly influence the magnitude of whole-limb vertical loading after ACLR.
There was less ankle dorsiflexion observed in the injured limb compared to the uninjured limb during the DVJ. Reduced peak ankle dorsiflexion and reduced ankle dorsiflexion at initial contact has been observed in the injured limb compared to both the uninjured limb and to controls during the DVJ.39,40 However, clinical measurement of maximum ankle dorsiflexion after ACL injury has been found to not differ between limbs.41 The combination of these studies may suggest that performance of the DVJ may not consistently engage physiological end-range dorsiflexion. Therefore, the difference between limbs in peak dorsiflexion after ACLR may be largely attributable to an avoidance strategy of the injured limb.40 In the current study, the observed combination of reduced peak ankle dorsiflexion angle and vGRF in the injured limb provides supportive evidence for this avoidance phenomenon.
The fatigue protocol elicited reduced hip moment contributions to peak TSM. The reduction in hip contribution may reflect decreased muscle force output from the hip extensors after the protocol at the instance of peak anti-gravity demand. Hollman and colleagues18 previously reported a 25% reduction in isometric hip extension strength after a similar protocol. The reduced hip contribution to peak TSM seen in the current study participants may reflect a transient reduction in hip strength from the protocol. This reduction in hip contribution may impact performance of other tasks after a proximal extensor fatigue protocol and thus be of interest to clinicians observing movement in a post-fatigued state. Interestingly, the knee contribution in the injured limb appeared to improve after the protocol. Previous studies have shown that the ACLR population demonstrates a decreased knee contribution to TSM during single limb landing.26 While the joint moment redistribution elicited by hip extensor fatigue would be transient, there may be potential rehabilitative and training applications to further explore.
The correlation between increasing knee contribution and time since surgery aligns with previous literature demonstrating increases in knee extensor strength over time after ACLR. Barford and colleagues found that over the course of rehabilitation a higher percentage of individuals developed adequate knee extension strength as measured by isokinetic limb symmetry index.42 Additionally, in a review of literature, it was recommended that it could take up to two years for knee extensor strength to recover.43 The average length of time since surgery of the current cohort was nearly five years, meaning they had greater opportunity to recover quadriceps strength. It is unknown if time since surgery and percent change in knee contribution would have a different relationship in those closer to surgery.
There are limitations to this study. As this is a cross-sectional study, it cannot be determined if the observed response to the hip extensor fatigue protocol would have been seen prior to injury. The sample size is relatively small and therefore prone to sampling error, a larger sample would improve the generalizability of the results to confidently characterize the ACLR population. While biomarkers of fatigue were not collected, participants performed three efforts of a validated single effort hip extensor fatiguing task. Trunk data were not collected, but could help explain the total body impact of the modified Biering-Sorensen fatigue protocol. Also, as with any study utilizing traditional motion capture techniques, skin movement artifact can affect the joint motion estimates.
Conclusion
The results of this study demonstrate that a hip extensor fatigue protocol elicits reduced bilateral hip extensor contribution to total support moment when anti-gravity demands are greatest in persons with ACLR. The clinical implications of this redistribution warrant further investigation.
Conflicts of Interest
The authors have no relationships/activities/interests to disclose that are related to the content of this manuscript.