INTRODUCTION
Sex differences related to neuromuscular control have been widely studied due to higher musculoskeletal injuries incident rates, such as anterior cruciate ligament (ACL) injury,1 ankle instability,2 and shoulder instability,3 in females compared to males. Females are two to four times more likely than males to sustain ACL injury when accounting for sport and activity level.4 Sex differences in anatomical structure, hormones, and neuromuscular control have been described to contribute to ACL injury risk.5 It has been widely reported that movement biomechanics and associated neuromuscular factors differ between sexes.6 Females show greater knee valgus,7 less knee flexion at initial ground contact,8 less muscle stiffness,9 and larger quadricep activation10 during jump landing tasks. One of the possible contributors to sex differences in neuromuscular control is proprioception. Proprioception, the sensory information arising from peripheral areas, influences neuromuscular control through its modulation of postural control, joint stability, and conscious sensation.11 Females are generally known to have diminished proprioception in comparison to males,12 especially when measured by kinesthesia (one’s ability to detect motion and direction).13 However, the underlying mechanisms responsible for the observed sex differences in movement patterns are poorly understood.
While much of the ACL literature has considered sex as a biological variable influencing dynamic movement and knee neuromuscular control,14 sex differences have only been documented peripherally using such tools as biomechanical analyses and electromyography.7 However, less is known about cortical contributions to sex differences for knee motor control. As the brain has an essential role in processing and integrating the sensory signals that arise from the peripheral areas to generate appropriate motor responses,15 sex differences in cortical activity may play a role in neuromuscular control variability. Neuroimaging techniques provide an avenue to identify brain function during movement to better understand neuromuscular control mechanisms. Functional magnetic resonance imaging (fMRI) is a neuroimaging technique that allows noninvasive measurement of human brain structure and function with high spatial resolution.16 Over the past few decades, upper limb fine motor control movement tasks have been widely studied with fMRI methods to better understand mechanisms of neuromotor control.17 However, fewer studies have observed brain function while performing gross lower leg movement tasks due to technical difficulties, including the need to minimize head motion. These studies have examined knee extension-flexion movements,18 pedaling,19 and unilateral leg presses,20 finding that brain regions including sensorimotor area, supplementary motor area, premotor cortex, cerebellum were highly activated during lower extremity movement tasks. Despite this research on brain activation during lower extremity motor tasks, less is known regarding whether males and females have differential cortical activity during lower extremity movements. There is only one previous study examining sex differences in brain activation during isometric ankle dorsiflexion in men and women.21 While Yoon et al.21 reported that young men and women have similar cortical activation of motor areas, this was limited to an isometric contraction. It is unknown if any studies have investigated sex differences in brain activity during dynamic knee joint actions. Therefore, the purpose of this study is to understand the differences in brain activation patterns between sexes during a simple active knee extension-flexion movement. Since females are reported to have poorer knee joint proprioception relative to males,12 It is hypothesized that females would have higher activation in the somatosensory areas as a compensatory strategy to sustain the same knee motor performance.
METHODS
Twelve males and seventeen females age eighteen to twenty eight, physically active at least two to three times a week, and right-handed/footed were recruited from a university population. Participants participated in running or cutting/pivoting activity as demonstrated on the Marx scale22 at least once a week. Participants were excluded if they had: a previous history of significant lower leg injuries and surgeries, any neurologic disorders, were currently undergoing a neuromuscular training program or had any contradictions to MRI assessment (any metal or implanted medical device in the body or claustrophobic, etc.). All participants read and signed an informed consent form approved by a University’s Institutional Review Board for the Protection of Human Subjects.
Functional Magnetic Resonance Imaging (fMRI)
All MRI data were obtained using a 3T Siemens MRI scanner with a 16-channel head coil (Siemens Tim Trio; Erlangen, Germany). Participants were placed on the MR scanner table headfirst and in a supine position. Head motions generated by lower extremity movement tasks can induce unwanted artifacts that interfere with fMRI data.23 Therefore, we spent considerable effort to minimize head motion by using a variety of restraints. Participants were stabilized with straps around their hips and chest, then sandbags and multiple sizes of pads were placed around the participant’s head within the head coil to minimize head motion. A mirror was placed on the head coil so that participants were able to see both their own leg and the researchers positioned in the adjacent operator room during the entire MRI scan.
Movement Task
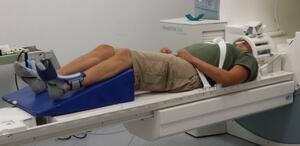
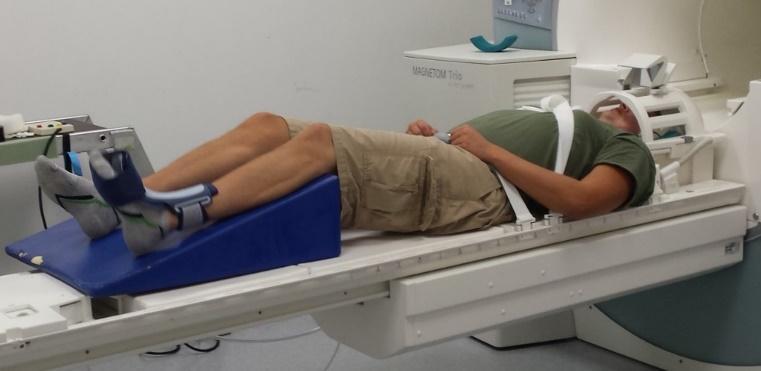
While obtaining functional MRI data, participants are required to perform knee extension flexion movement task. A bolster was placed underneath the participant’s leg to allow approximately 45 degrees of knee extension flexion (Figure 1). An ankle immobilizer was positioned on the left ankle to ensure isolated knee extension-flexion movements during the functional imaging tasks (Figure 1). Instruction was given to participants to perform left leg knee extension-flexion movements with a metronome (1.2 HZ) following the auditory cue from the researcher to “start” and “stop”. During the movement task, participants relaxed for 30 seconds then performed 30 seconds of continuous knee extension-flexion exercise of the left leg followed by 30 seconds of relaxation. The participants complete four cycles of movement and relaxation. The auditory metronome was heard by the participant during the entire duration of fMRI scan to control rate of knee extension-flexion movements. The participants performed the task with only the weight of their own limb. There was a familiarization session prior to the scan.
fMRI data acquisition
The structural and functional neuroimaging were collected following the methodology of a previous fMRI study by Raisbeck et al.24 Structural images were initially obtained (repetition time = 2000 ms; echo time = 4.58 ms, matrix field of view = 256 mm; voxel size = 1 mm x 1 mm x 1 mm; scan time=6.5 mins). Then, functional magnetic resonance images were measured to attain the blood oxygen level-dependent (BOLD) signals during knee extension-flexion exercise. Functional image data (fMRI) were obtained (repetition time = 3000 ms; echo time = 28 ms, Flip angle = 78 deg; phase encoding direction = anterior to posterior; matrix field of view = 220 mm; voxel size = 2.5 mm x 2.5 mm x 2.5 mm) during the movement tasks.
fMRI analyses
A block design was used for the experimental tasks that include rest and knee movement blocks. It measured 10 full-brain datasets for each 30 seconds block, which resulted in 40 full-brain images for knee extension-flexion movements (4 blocks) and 50 full-brain images for rest (5 blocks); a total of 90 full brain images, congruent with previous work.24 MRI data were analyzed using the fMRI of the brain (FMRIB) software library (FSL: The Oxford Centre for Functional MRI of the Brain, Nuffield Department of Clinical Neurosciences, University of Oxford, Oxford, United Kingdom).25 Standard processing was completed for each subject’s data, including image format converting, reorientation, and brain extraction (using FSL BET).
Then, FEAT (sub-component of the FSL software) was used to perform pre-processing. The pre-process includes motion correction (MCFLIRT), interleaved slice timing correction, spatial smoothing at 6 mm full width at half maximum (FWHM), 4D mean intensity normalization.25 The Independent Component Analysis-based Automatic Removal of Motion Artifacts (ICA-AROMA) was used to remove motion-related noise and increase sensitivity to group-level activation.26 Then, the first-level analysis was performed for subject-level contrast (rest vs knee movements) using a cluster-based threshold with z threshold at 2.3 and p threshold at 0.05.25 This process also includes temporal filtering (90s).25
Finally, the higher-level analyses were performed with FLAME stage 1+2 using unpaired samples t-test to contrast between sexes (Female > Male; Male > Female) with a z threshold of 2.3 and p<.05 Gaussian random field cluster corrected.27 To avoid possible differences in brain structure between sexes that may can lead to misinterpretation of functional results, voxel-wise gray matter volumes were included as covariates during the higher-level analysis.28 Regions of brain activity were identified based on FSL tool atlasquery with Juelich Histological Atlas, Harvard-Oxford Cortical Structural Atlas, and Cerebellar Atlas in MNI152 space after normalization with FNIRT. Featquery was used to calculate a mean percentage signal change for each individual’s FEAT results within a cluster mask images from the higher-level analysis.
RESULTS
Demographics of the female and male groups are presented in Table 1. There were no significant differences between sexes in BMI (p=0.77) and Marx (p=0.32) physical activity scales (Table 1). Additionally, there was no significant difference in absolute (p=0.52) and relative (p=0.94) head motion between sexes during the experimental tasks (Table 1).
The fMRI comparisons between sexes are reported in Table 2. During repetitive knee flexion-extension movements, females demonstrated higher BOLD signals in right premotor cortex (p=0.008; Table 2; Figure 2A), the visual cortices right V3, V4 (p=0.011; Table 2; Figure 2B) and Left V1, V2 (p=0.004; Table 2; Figure 2C) Juelich Histological Atlas among the entire brain. The same regions also represent precentral gyrus, lateral occipital cortex, and intracalcarine cortex in the Harvard-Oxford Cortical Structural Atlas. Males demonstrated significantly greater activation in the right cerebellum compared to females with the peak voxel right VIIIa and VIIb (p <0.001; Table 2; Figure 2D).
DISCUSSION
Given the importance of sex as a biological variable in the study of neuromuscular control, the differences in brain activation between sexes associated with a simple knee flexion-extension task was examined. The results demonstrated that females had greater activation in the premotor cortex and the visual cortices compared to males during a voluntary knee extension-flexion task. Males had significantly greater activation in cerebellum.
Premotor Cortex
The premotor cortex plays an essential role in the planning or programming of voluntary movements.15 It has been reported that neurons in the premotor cortex begin firing about 800ms prior to voluntary movement.29 The premotor cortex also activates when receiving an instruction to move.30 During the experimental tasks in the current study, participants were given the auditory cues to begin lower limb movements and relax. Higher activation in the premotor cortex in females may indicate that females required greater resources dedicated to the planning of movement for even simple leg extension and flexion movement compared to males.
Activation in the premotor cortex also correlates with increasing complexity of targeted movements,31 especially the complex sequential movements.32 The current study movement task is involved with sequential knee extension-flexion exercise with rhythmic timing. Since females typically have a lower hamstring to quadriceps muscular ratio33,34 and decreased muscle strength of the lower extremity,35 potentially indicating a lower capacity for the knee extension-flexion movement task resulting in it being relatively more complex to regulate for females than males.
Visual Cortex
The results also demonstrated that females had significantly higher activation in their visual cortices. The visual cortex has a primary role in visual processing.15 The visual system is crucial to execute desired physical movements, especially in coordination, regulation, and control of movements.36 The finding of visual cortex activation was likely related to the ability of participants to see their leg during the tasks through the mirror located on the head coil. However, this activation was significantly higher in females compared to males. Previous work had reported that females demonstrated diminished postural stability compared to males, especially when visual perception was impaired.37 In addition, females show decreased proprioception compared to males.11,12 This may suggest that females rely more on using visual information in order to execute lower extremity motor tasks, potentially secondary to increased visual cortex activity to generate postural corrective knee movements.
Specifically, the results revealed significantly more activation in visual cortices V1, V2, V3, and V4 in females when compared to males. The primary visual cortex (V1) is the first stage of processing visual information that receives visual input from the retina.38 The secondary visual cortex (V2) processes visual stimuli and illusory contours.39 V3 plays a critical role in transmitting visual information, especially processing motion, from the primary visual cortex to parietal and temporal cortices.40 V4 is interconnected with the higher-order cortex transferring object and spatial visual information.40
The Harvard-Oxford Cortical Structural Atlas also reports that subregions in the visual cortex includes the intracalcarine cortex, lingual gyrus, occipital fusiform gyrus, and lateral occipital cortex. The intracalcarine cortex and lingual gyrus are a part of the primary visual cortex,41 and they contribute to the process of visual stimuli.38 The occipital fusiform gyrus is located in the occipital lobe, and is associated with perceiving body parts and their actions.42 The lateral occipital cortex is also responsible for visual shape processing.43 The lingual gyrus also known as the cross-modal cortex has a high capacity for neuroplasticity when experiencing loss of sensory input.44 Sensory information, including vision, proprioception, and vestibular systems, all have a demonstratable impact on proper motor system function.45 When proprioceptive information is deficient, vision and vestibular systems may become more highly engaged in order to carry out motor function. Therefore, impaired proprioception in females may alter the cortical function in order to increase neural activity in the visual cortices as a potential compensatory strategy. This compensatory strategy may contribute to females relying more heavily on visual information to perform motor tasks and contribute to sex differences in neuromuscular control. Since the findings suggest that females may rely more on utilizing visual information during physical movement, visual-motor training additions to injury prevention training may be particularly efficacious for females.
Cerebellum
Males displayed higher cerebellar activation in lobule primarily VIIIa and VIIb as well as VIIIb and IX of the right cerebellum compared with females. The cerebellum coordinates voluntary movements, motor control, muscular coordination, and executive function.15 The lobule VIIIa receives projections from the primary motor cortex,46 and the lobule VIIIa and VIIIb represent sensorimotor function.47 With regard to lobule VIIIa and VIIIb function, previous work has demonstrated increased activation of these areas in the upper limb compared to lower limb motion in a female only population.47 The lobules VIIb and IX are associated with executive functions, including working memory, planning, organizing, and visual divergent thinking.48 The cerebellum is also engaged with voluntary movement with event timing.49 O’Reily et al.49 showed a significant cerebellum activation during perceptual prediction task when only temporal information (velocity) is involved to predict, but not spatial information (direction). During movement task used in the current study, there was a metronome to assist participants in performing extension-flexion movement with the same timing. Thus, the results of higher activation in the cerebellum in males may indicate that males have a heavier cortico-cerebellar strategy during motor control than females, especially when the task was involved with precise timing. However, the relation to potential injury risk is unknown at this time.
Sex Differences in Brain Function and Structure
According to the best-known available data, there is limited research of sex differences in brain activation during lower limb motor tasks. Yoon et al. assessed brain activation patterns in males and females during isometric ankle dorsiflexion with various force control.21 They discovered that most of the motor cortex areas were activated similarly, with the exception of the right inferior temporal gyrus having greater activity in males at 70% of maximal voluntary isometric contraction. The inferior temporal gyrus plays a primary role in visual stimuli processing, objects recognition as well as biological motion processing.15 While this previous finding does not support current results, significant differences in task (isometric vs. isotonic), intensity (70% MVIC vs. body weight), and joint (ankle vs. knee) may confound direct comparisons to current work that the visual cortex area was highly activated in females than males.
While sex differences in brain function during lower limb motor control is not well studied, investigations into sex differences during the upper limb fine motor tasks have been reported.17 Females demonstrated generally higher cortical activation than males during finger tapping tasks.17 These highly activated regions included the parietal, superior temporal, motor, and somatosensory regions, in addition to the middle occipital cortex. Males displayed higher cortical activation of the caudate nucleus and basal ganglia, as well as the fronto-parietal and temporal regions.17 Lissek et al.17 suggested that there may be a different aspect of the motor-related cortical process between sexes. Thus, a sex-specific functional cerebral organization may be used to achieve the same motor skills. Sex differences in brain structure and structural connectivity are also well understood and may contribute to differences in functional activity. Gur et al.50 found that males have increased white matter size and spinal fluid. Males also have relatively larger cerebrum and ventricle volumes, whereas females were found to have larger overall cortical volumes.51 Moreover, males reveal higher communication within the hemisphere, and females show higher interhemispheric communication.52 Even though brain function and brain structure are measured differently, the previous and current results support the differences in the brain between sexes. Thus, while work is limited in scope, there is support for sex to be considered as a biologic variable when performing research involved in understanding central activation during motor tasks.
The current study results revealed that females showed higher activation in the premotor cortex and visual cortices. This may be due to the fact that females require greater cortical resources to plan and execute motor movement and exhibit less proprioception than males. Furthermore, males demonstrate heavier cortico-cerebellum strategies than females, especially when precise timing is involved with the movement task. These findings may help practitioners and clinicians develop training and rehabilitation methods that improve the efficacy of using visual and sensory information in females. There are a few studies utilizing a visual resources, such as virtual reality system and visual biofeedback to train neuromuscular control in order to induce movement adaption to decrease injury risks.53–55 Thus, rehabilitation methods using visual resources may help train female athletes to rapidly pre-plan/program movements in response to changing stimuli, thereby decreasing the risk of injury.
Limitations must be considered when interpreting the current study. There was a relatively low sample size (N=29, male=12; female=17) in this study. In addition, there was a significant age difference between groups (male=22.8±2.2; female=20.6±1.6, p=0.004). However, age was controlled in this study by limiting participants age to between 18-28 years old. Despite well know effects of aging on brain function,56 an age difference of just a few years would likely have a minimal effect on the results, thus the impact of the age difference to our results was not a major concern. It is also important to note that the cortical activation differences may not be due to sex differences but instead other factors that may inherently differ by sex (structural differences). To that end, physical activity level was controlled in this study by recruiting only participants who were physically active in order to minimize the impact of confounding variables. In addition, the movement task was standardized to individual’s body size by performing knee flexion-extension movements with participants’ own limb weight.
CONCLUSION
Results of the current study revealed that females have higher neural activation in the premotor cortex and visual cortices compared to males during active knee extension-flexion movements. Males demonstrated significant higher activation in the cerebellum than females. The results, as well as previous work17 reporting sex differences in brain activity during motor tasks suggests the need to include sex as a biologic variable in neuroimaging studies involving motor tasks. Understanding sex-specific brain function during an equivalent lower limb motor task may shed further light on sex differences in lower extremity neuromuscular control.
Acknowledgments
The results of this study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation. There are no conflicts of interest or funding associated with the present study.
Conflict of Interest
The authors declare no significant competing financial, professional or personal interests that might have influenced the presentation of the work described in this manuscript.
_.png)
_.png)