INTRODUCTION
Female participation in elite sport has increased significantly in the last 10 years.1,2 Athletes returning to sport post-partum is also more common. Limited evidenced-based protocols, established time frames or injury risk data exist to guide the post-partum athlete’s return to sport (RTS).2,3 Athletes have a higher risk of pelvic floor dysfunction (PFD) than non-athletes (54% versus 7%)4–6 and there is also an increased prevalence in post-partum women compared to nulliparous women (35% versus 2.8-7.9%).5,7,8 It is suspected that there is an increased risk of PFD for post-partum athletes and therefore there is a need to identify best-practice for RTS in this population.
There are mixed reports of the optimal timeline for returning to sport post-partum. A recent editorial advocates for a multidisciplinary, biopsychosocial injury rehabilitation model for postpartum return to high impact activities yet such a model has not been investigated.9 Evidence suggests that 71% of athletes return to training within six weeks post-partum.10 Similarly, survey data indicates that 40% of post-partum recreational runners return to running at four weeks11 and 78% at 12 weeks.12 Contrary to this, guidelines based on expert opinion for return to running identify potential risk factors in returning to running in the first three months post-partum.8 The prevalence of urinary incontinence (UI) or vaginal heaviness was reported by more than 30% and musculoskeletal pain by 84% in early post-partum runners.12 Urinary incontinence increases the risk of having musculoskeletal pain with running (RR 1.97 95% CI (1.37, 2.84)12 and negatively influences performance and participation.13 Additionally, authors have shown reduced abdominal wall strength post-partum irrespective of delivery mode which may contribute to poor trunk control, PFD and suboptimal RTS.14 Given the high rates of PFD and pain in post-partum women returning to exercise and the influence on performance, there is a clear need to evaluate PFD within a global neuromuscular assessment prior to RTS.
The purpose of this case report is to detail the management of an elite athlete who presented following cesarean section (CS) with the goal of RTS within 16 weeks. It draws on the current sports literature of physical and psychosocial factors that influence successful RTS15,16 and factors specific to post-partum RTS, referred to herein as the “mother load”.17
CASE DESCRIPTION
GT, a 27-year-old primiparous caucasian professional netballer presented at four weeks post-CS for RTS screening and assessment of pelvic floor muscle (PFM) function. Her healthcare team included a sports physiotherapist, performance manager, coach, dietician, psychologist, obstetrician, sports physician, and pelvic health physiotherapist (author). GT consented to this case being published.
Patient History
GT had been playing elite netball for 10-years and had no time off training or playing due to injury. Prior to childbirth, she had no prior urinary or anal incontinence, obstructive emptying or sexual health concerns during sport or daily life.
GT reported coping well throughout pregnancy and in the acute post-partum phase. Her weight gain during pregnancy was within normal range (11.5-16Kg) based on her pre-pregnancy body mass index.18 She experienced minimal medical, musculoskeletal, or pelvic health issues during pregnancy and continued to train non-contested with the netball team until 30 weeks gestation, remaining physically active until birth in line with current guidelines.19,20 She did not complete pelvic floor muscle training during pregnancy. There was no other relevant medical history.
GT underwent an elective low segment CS with standard wound closure due to breech presentation.17,21 There were no peri-operative or post-operative complications for mother or infant. Lochia stopped at three weeks post-partum. Breastfeeding was established on day four with no latching concerns, nipple, or breast discomfort. GT and infant were discharged on day five, a standard length of stay in an Australian private hospital. GT reported having a small pre-pregnancy breast size and an insignificant size increase.22 GT reported being well supported personally and by her team and team support staff. She reported her post-partum weight at her four-week initial review was 5Kg greater than pre-pregnancy.
At four weeks post-CS, she was walking 30 minutes daily at a casual pace and performing two sessions per week of three sets of light intensity unweighted squats and lunges (rate of perceived exertion 2-3/10).20 There was no itching, discolouration, or discomfort around the scar.21 She did not report any symptoms, nor bother related to her pelvic floor function.23 She had not returned to sexual activity. She reported general fatigue and an average of six to eight hours accumulated sleep per night. When asked, GT identified some concerns with RTS: “birth can cause pelvic floor muscle injury and prolapse and that it can get worse if you do the wrong thing”.
Patient Goals
-
Play in round one of the national competition at 16 weeks post-partum
-
Assess her individual PFD risk
-
Commence pre-season training at 12 weeks post-partum
-
Continue to exclusively breastfeed for the first six months post-partum
Evaluation
GT’s history suggested a low predictive risk of PFD16,24 confirmed by the validated Australian pelvic floor questionnaire (APFQ) (Appendix A).23 Psychosocial screening evaluated factors known to influence RTS and post-partum wellbeing including post-partum depression and anxiety, sleep quality, readiness to return to sport, and fear of movement scales (Table 1).9,15,17
Early post-partum physical performance tests were compared to pre-partum data (Appendix B) provided by GT’s sports physiotherapist, and which were relevant to early intervention (Table 2). Evidenced-informed screening for high level neuromuscular performance in netball8,15,29,30 was planned to be completed by the sports physiotherapist at eight weeks post-partum. A summary of key examination findings is found in Tables 1 and 2.
Diagnosis
GT did not meet diagnostic criteria for PFD such as urological or anorectal disorders, prolapse or pain syndromes31 or diastasis rectus abdominis (DRA).14,27 Given that the definition of PFM dysfunction requires patient concern and alteration in normal pelvic floor muscle function,31 GT’s examination identified sub-clinical alterations in PFM:
-
Neuromotor deficits
-
delayed recruitment (voluntary and functional)
-
delayed relaxation
-
-
Reduced endurance with sustained and rapid contractions
-
Reduced power during a rise in intra-abdominal pressure (IAP)
Musculoskeletal screening identified reduced rectus abdominis strength (as per previously published testing guidelines),14,28 reduced lower limb strength compared to her pre-partum data (Appendix B) and reduced stationary balance testing of < 30seconds.
Biopsychosocial screening identified changes commensurate to four weeks postpartum9,17 including:
-
Moderate fear of movement
-
Belief that all women are at risk of PFD post-partum impacting her ability to RTS.
Intervention
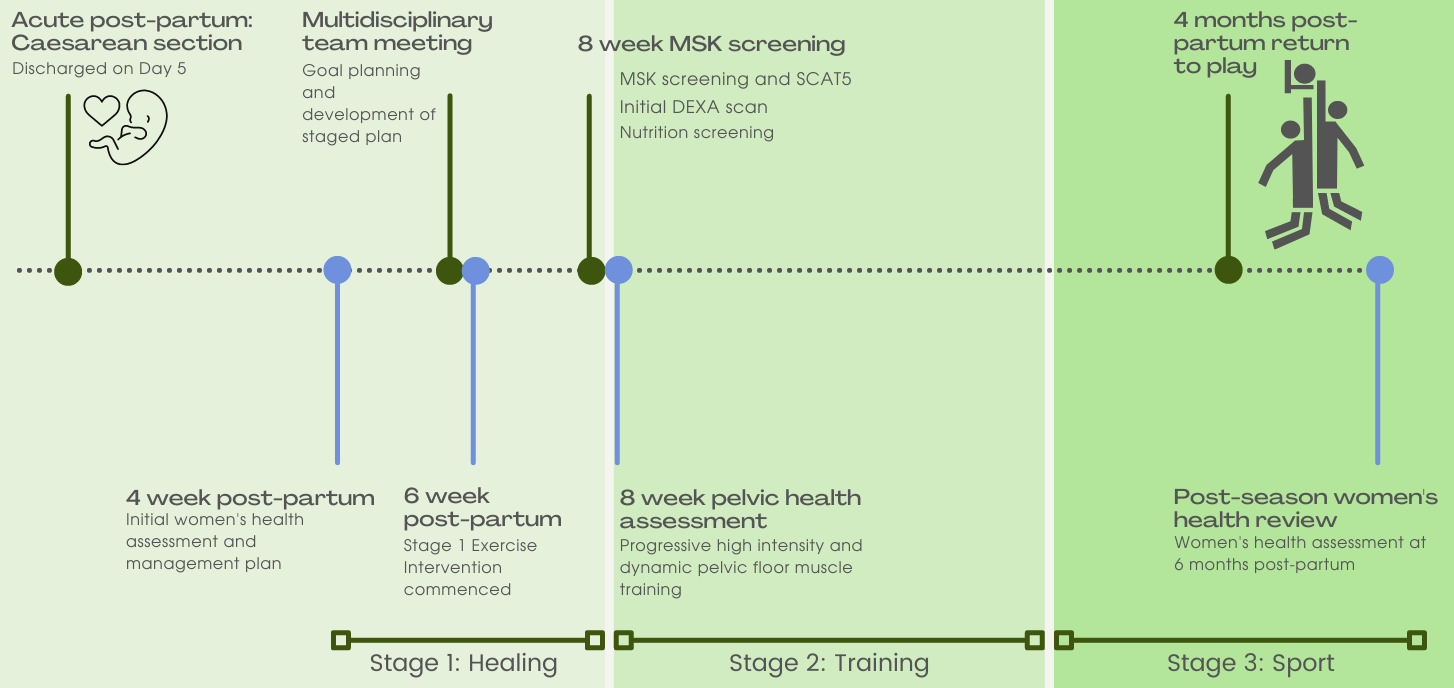
Stage one of GT’s pelvic health physiotherapy management started in the immediate post-partum phase2 as part of a collaborative and criteria based RTS model (Figure 1).8,15,29,30,32 During this stage, the pelvic health physiotherapist directed case management.
Management was informed by time-based wound healing principles17,21 and criterion-based assessments such as asymptomatic response to exercise testing.15,20,30,32 During all stages, the patient was asked to keep a diary to record training, breastfeeding, sleep, and symptoms. Exploratory interviews with post-partum athletes suggest that social support, childcare, and training specificity are all enablers of successful return.9,17 The shared-decision and development of GT’s netball-specific exercise program considered child minding, caring needs, lactation, and GT’s availability while targeting optimal training dosage.29,32
Stage 1: Proliferative healing and preparation (up to 8 weeks post-partum)
The focus of pelvic health physiotherapy intervention in Stage 1 was:
-
multidisciplinary engagement
-
education to reduce fear of movement and address PFD beliefs
-
sleep optimization
-
wound healing
-
PFM rehabilitation
Obstetrician clearance to return to a graded training schedule was given at six weeks post-partum. Nutritional and psychology reviews were initiated given their link to UI2,16,17 and RTS readiness, performance, and sleep.11,15,17 DEXA scans were undertaken as per the team’s medical protocol for all athletes. For GT, this enabled monitoring of unattenuated axial bone loss associated with lactation and the consequent increased risk of bone stress injury.10,17
Individualized education sessions provided clear, simple and evidence-informed knowledge that a CS is unlikely to result in pelvic floor muscle injury.12,24 This was facilitated by a lecture-style and teach-back approach that outlined the neuromuscular changes that occur in pregnancy and compare and contrasting modes of delivery. During the teach-back discussion and when asked GT reported reduced fear of PFM injury with RTS. Other major themes of generalized education were sleep optimization,8,17 feeding postures, bra considerations,9,17,22 and timing of physical activity around expressing.2,17
Relative contraindications to early post-partum exercise were increasing longitudinal wound tension and wound pain (> 4/10).11,21 In the immediate post-partum phase, there is minimal scar integrity (< 30% tensile strength).21 Scar dressing with silicone tape and daily massage to reduce hypertrophic scarring21 were taught and patient self-efficacy confirmed. Abdominal wall compression wear during activity was recommended8,21,33 given it may be more effective than core-based exercise in the immediate post-partum phase for pain intensity and perceived function.33 Abdominal wall exercises were not specifically given, and wound pain was used to guide her response to general exercise. To minimize longitudinal scar tension, verbal and visual instructions were given to avoid trunk rotation and limit cross-body exercises.
There is insufficient evidence in pelvic floor rehabilitation guidelines regarding pain rating and training load early post-partum.8,11 In early post-partum studies, women reported no pain from commencing PFM exercises immediately post-birth, therefore early PFM training was commenced.34
GT’s PFM training program (Table 3) was developed in line with exercise prescription15,29,32 and PFM training guidelines8,20,26,30,35,36 to target GT’s impairments and train specifically for the characteristics of netball.15,29,37 Netball requires endurance and repeated explosive effort (power). Athletes involved in impact sports have been shown to have, and require above, normal PFM strength38 to withstand the increased IAP that occurs with running and jumping.2,4,6,12,36,38 The aim of this PFM training program was to achieve hypertrophy,26,35 speed of muscle recruitment during explosive tasks36 and endurance.2,26,35 Verbal feedback, digital palpation and patient verbal confirmation of awareness were used in all prescribed postures to facilitate a correct technique.35,36 Early training (week 4) focused on repeated daily exposure to achieve neural adaptation with normalisation of motor recruitment and relaxation. Initial intra-vaginal pressure biofeedback (Peritron®, discontinued, Cardio-Design, Victoria) was conducted to guide correct sub-maximal voluntary contraction and relaxation at her first session. Progressive daily PFM training has been shown to be effective in addressing PFM dysfunction26,35 with greater benefit from a combined strength and neuromotor program (Table 2, week 6).39 GT was shown how to self-progress her repetitions and postural load if the quality of PFM contraction was maintained. Progressive overload was achieved by increasing repetitions or increasing load by modifying task to challenge IAP demands.30,32,40 Recommendations were given to perform the strength-based exercise earlier in the day and the endurance-based exercise later in the day to allow for sufficient recovery.15 However, a shared-decision making approach was taken to optimise the likely times of exercise completion around breastfeeding and other childcare needs. Other strategies included metronome pacing and an activity diary to facilitate adherence.39
Stage 2: Return to training (from 8 weeks post-partum)
At her week eight assessment, GT had reduced abdominal strength compared to pre-partum. With wound and abdominal fascial strength continuing to increase to 80% at 12 weeks,2,8,17,21 graded abdominal strengthening and trunk rotation activities were permitted, with continued scar taping21 and symptom monitoring. Insufficient evidence currently exists for best-practice abdominal wall strengthening post-partum.14,41 All women post-partum, with and without a diagnosis of DRA, have reduced abdominal wall strength compared to nulliparous controls, however the importance of this remains unknown.14,41,42 Expert opinion supports a focus on integrated abdominal wall control compared to isolated muscle strength variables.43 In consideration of an integrated approach to rehabilitation, initial abdominal wall exercise was prescribed as isometric lower abdominal wall activation in supine and quadruped postures as outlined in Berg-Poppe et al41 and progression principles were applied as outlined by Christopher et al.30 Given the lack of specificity identified in the literature for post-partum abdominal wall rehabilitation,41,42 exercises were progressed when able to achieve five sets of 10, with 15-second recovery periods as based on abdominal wall training guidelines for sporting injuries.32 Quadruped exercise was progressed to add in upper limb movement once perceived as easy by GT and confirmed by the physiotherapist that there was minimal trunk deviation, no compensation, doming or breath-holding during the task. Static exercise such as plank and side plank were progressed from knee based to full versions using the same criteria of observed quality and once achieving one minute endurance, five repetitions. The abdominal wall program was completed three non-consecutive days per week.
The PFM training program was progressed to focus on power training and netball-specific dynamic exercises to induce pre-active and supra-maximal PFM contraction (Table 3).35–37 This included PFM pre-activation with a cough, a five-meter chest pass at rapid speed, lunge to knee drive, and progression from double to single leg jumping to volitional failure.36 Throwing distance, weight, IAP demand and hop distance were progressed based on symptom monitoring and perceived levels of exertion.29,30,32,40
Having attained normal PFM function and being asymptomatic during Stage 1 functional testing, GT commenced a return to run program at 10 weeks post-partum.29
Stage 3: Return to Sport (14 weeks post-partum)
Ongoing PFM training was integrated into GT’s conditioning sessions to maintain function following the 12-week training program. Her program included PFM control during multi-directional stepping/hopping to volitional failure and PFM endurance.36 GT was prescribed PFM training three times per week to align with exercise prescription guidelines for rest and recovery given her high intensity training load at this stage.9,15,32,37 Abdominal wall exercise included cross-body control with load and progressive balance demands, and ongoing plank and quadruped exercise. Scar taping was ceased. Throwing and catching drills with reaching outside base of support provided ongoing challenge for abdominal function.
Follow-up and Outcomes
The primary outcome was successful return to full match play at 16 weeks post-partum. Reassessment at six months post-partum identified improved PFM function. There was no injury reported during the season. GT continued successfully breastfeeding. As little is known about early post-partum recovery and the influence of high-impact activity,2,8,17,44 a six-month review identified no de novo PFD. A critical component of GT’s successful RTS was communication between all stakeholders and consideration of all biopsychosocial factors.9,17 GT provided her perspective on RTS: "It has really helped having Netball (state) and the state team, they’ve been amazing." Her satisfaction with intervention: "It was really well planned out for me; it was very doable".
DISCUSSION
Current post-partum guidelines do not provide clear criteria to evaluate a post-partum athlete prior to RTS, however several criteria have been suggested when returning to sport following injury: pain/symptom free clinical evaluation, minimal range of motion/strength deficits, sport specific functional field testing, and no apprehension during full effort.10,15,29 These criteria can be applied in pelvic health to evaluate a holistic post-partum RTS management plan.
Clinical evaluation for post-partum RTS should include screening for PFD risk factors. Pre-existing PFD risk factors include: pre-pregnancy symptoms, prior vigorous exercise, depression and recurrent urinary tract infection (rUTI).7,24,25 Most significantly, pre-pregnancy symptoms (and severity) are associated with a 15-17 times increased risk of persistent UI or urgency24 and these are the most prevalent symptoms in athletes.6,24 In a multivariate analysis, vigorous exercise (> 4 times per week), depression and rUTI were all associated with a 40-200% increased risk of post-partum PFD.16 While exercise status correlates with greater risk of developing symptoms, recent studies identify a two- to three-fold increased chance of earlier return to running with higher weekly running volumes and running during pregnancy.11,12,44 Therefore, both volume of exercise and the presence of PFD need to be evaluated. The author suggests that ante-natal screening for PFD, knowledge of an athlete’s exercise habits, and the use of a validated PFD screening questionnaire23 may identify risk factors as well as identify athletes antenatally that would benefit from assessment and intervention. In GT’s case, an asymptomatic history was an enabling factor for RTS.
Fascial support and pelvic floor resting position have a direct influence on urethral closing pressure and the development of PFD.26,38 Tissue distensibility increases during pregnancy irrespective of delivery mode.45 Many studies identify increased rates of UI, POP and anal incontinence (AI) with longitudinal studies reporting birthing mode as an independent risk factor.24 While CS has been shown to be protective for the incidence of urinary symptoms and prolapse (POP),7 pregnancy may change the risk profile during the immediate post-partum phase for an asymptomatic athlete, sub-optimal function should also be addressed to achieve RTS. In relation to CS birth, there is no significant difference of pelvic floor morphology and bladder neck mobility during pregnancy and post-birth with a return to pre-pregnancy values observed.45 Women experiencing vaginal heaviness were 50% less likely to return to running and this correlated with pre-pregnancy urinary symptoms, rUTI and vigorous exercise.11,12,24 Wound healing,21 self-selected timing of RTS,17 higher levels of fatigue,17 nutritional demands17 and post-operative pain2,12,17 may be a reason for a CS not being an enabling factor for return to running11 despite being a lower PFD risk.16,24 An additional factor can be the influence on abdominopelvic control. Current research suggests that abdominal wall strength and control remains reduced at 26 weeks post-partum irrespective of activity levels or type of birth.14 Unfortunately, the mixed quality and heterogeneity of the research has provided low certainty as to the type and benefit on improving function of the abdominal wall with post-partum rehabilitation.14,33,41,42
Athletes have been shown to have UI despite strong PFM, whereas increased bladder neck descent (BND) was a risk factor for UI.4,25,26,38 Similarly, excessive lengthening of the urogenital hiatus over time is an independent risk factor for UI and POP and may be related to altered perineal muscle function, repetitive high IAP as well as vaginal birth.7,25,45 Early return to heavy work post-partum has been associated with a three-times increased risk of POP symptoms.44 However, women who return to high-impact exercise early post-partum have no difference in PFM strength, reported UI or POP symptoms compared to non-exercisers at six weeks or 12 months.44 Interestingly, intensity and long training hours of athletes correlate with the onset of PFD16 suggesting chronic overload may be a risk factor versus exercise itself. This may place an athlete with a full-time training load at greater risk of developing PFD on RTS. This case study evaluated urogenital hiatus and bladder neck descent using 2D transperineal ultrasound at rest and under load, performed longitudinally to monitor structural change.25,26 A consideration for future studies would be pre- and post-training assessments of tissue distensibility and whether this correlates to the development of symptoms and influences RTS. On reflection regarding this case, earlier engagement of a pelvic health physiotherapist prior to pregnancy would have allowed better planning in the post-partum phase.9 In future practice, the acute post-partum phase (up to six weeks) would be advocated as a time for tissue restoration and building psychosocial resilience. Further multidisciplinary planning would be beneficial to include technical skills/drills in early stages of rehabilitation in a low intensity and controlled environments.
Optimizing neuromuscular pelvic floor function can improve urethral closing pressures and the levator plate position.26,31,35,39 PFM strength, timing, and coordination are highly correlated with UI,26,35,36 and vital to support IAP loads during running and landing in netball.2,4,16,26,40 Impaired reaction times are associated with a three-fold risk of UI.36 There is grade 1 evidence for PFM training in the general population,35 and sport and training specificity should be considered when prescribing PFM training.4,15,46 The authors acknowledge the limitation of the sport specific training protocols provided in this case and support future work to provide multi-disciplinary RTS protocols. The author suggests promotion and engagement with stakeholders and sporting decision-makers to create greater opportunities for primary pelvic health screening in female athletes.
The lack of PFM functional measures that offer predictive validity of pelvic health risk and RTS was a clinical challenge in this case and the research is underdeveloped compared to other rehabilitation protocols.4,15,30 While not validated, the author considered symptom responses during jumping to identify any issues prior to the introduction of sport-specific training. There is currently limited evidence for the prevention of PFD with PFM training. Specific ante-natal PFM training has been shown to reduce the risk of developing UI by 62%,47 however, research in general sporting populations would suggest targeted training does not prevent injury.15,29,32 Further research into the prognostic value of PFM functional field testing may assist safe RTS.
CONCLUSION
A postpartum athlete experiences the “mother load” of perinatal musculoskeletal and physiological changes as well as experiencing the demands of early high intensity training. Current evidence suggests PFD has a significant influence on returning to exercise and that athletes are at greater risk of PFD. The RTS timeline in GT’s case is limited to an asymptomatic woman following CS returning to netball. This case study proposes a battery of tests to detect PFD and integrates pelvic health into a RTS program. Multidisciplinary planning should begin during early pregnancy and include a pelvic health physiotherapist. A range of factors should be considered, PFD, delivery, wound healing, sleep, breastfeeding, and breast changes. Future areas of research include developing post-partum RTS protocols, diagnosis, prevention, and management of PFD in athletes and the longitudinal effects of PFD and RTS.
Financial Disclosures
The author does not have any financial or personal relationship with people or organizations that may inappropriately bias the work.
Acknowledgements
The author wishes to thank Dr Kerrie Evans PhD, MHealthSc(ManipPhty), BAppSc(Phty), FACP, GAICD and Dr Margaret Sherburn PhD, MWmnsHlth, BAppSci (Phty).
CONFLICTS OF INTEREST
The author of this paper has nothing to disclose.