INTRODUCTION
Acromioclavicular joint (ACJ) separation injuries are commonly seen in athletics. Although there is a paucity of recent evidence on the epidemiology of ACJ separation, the most recent data suggests that these injuries account for up to 53% of athletic related shoulder injuries.1 Interestingly, concomitant intra-articular joint pathologies (e.g., labral tears, supraspinatus tears, inferior glenohumeral ligament tears) have been reported, ranging from 14-77%.2–5 Because multiple sites and tissues can be affected, such injuries can be regarded as complex shoulder injuries. Labral tears and supraspinatus tears have been identified as two of the more common intra-articular pathologies associated with ACJ separation.2–6 Interestingly, the incidence of concomitant labral tears associated with ACJ separation seems to be linear, and ranges from 60% in grade 1 ACJ injuries to 100% in grade 4 ACJ injuries.4 Research investigating conservative non-surgical treatment (physical therapy/cortico-steroid injections) of these types of complex shoulder injuries is scant, with most quality trials on shoulder pain addressing the more encompassing diagnosis of subacromial impingement syndrome.7–10 This seemingly may be an important barrier to effective comprehensive management of a complex shoulder injury, as each injured tissue likely needs to be addressed independently at specific time frames for optimal outcomes.
Intuitively, there seems to be a need for novel point-of-care strategies to identify and promote specific tissue healing at each pathoanatomical site of a complex shoulder injury, while improving functional outcomes in a safe/timely manner. Orthobiologic medicine complimented with appropriate regenerative rehabilitation may be an attractive option. Platelet-rich plasma (PRP), and prolotherapy are two common therapies used within the spectrum of orthobiologics/regenerative medicine.11,12
PRP is a preparation of autologous plasma enriched with a platelet concentration above that normally contained in whole blood.13 Following exsanguination, it is generally prepared in a centrifuge to separate its contents for a greater concentration of platelets within the blood. It is within this concentration that the platelets are extracted and re-injected into specific areas of musculoskeletal injury. Once injected, the PRP exerts a strong bioactive effect, releasing a plethora of key growth factors that promote healing.14 The overall goal of using PRP is to have supra-therapeutic platelet concentration in a small volume of plasma, in order to promote healing of tissues (joints, cartilage, tendons and ligaments) that have intrinsically poor healing capacity.13 Further, preparation of blood-based orthobiologic compounds such as PRP may be enhanced with specific exercise prior to exsanguination.15,16 Unfortunately, the use of PRP for the management of shoulder conditions is not without controversy.17,18 However, recent evidence has suggested that PRP results in short and long term improvements in pain and function in patients with partial rotator cuff tears.19–21 Only one case series has suggested improved outcomes treating the glenoid labrum with PRP.17,22 To date, there seems to be minimal evidence supporting the use of PRP in conservative orthopaedics, with a strong recommendation for standardization of PRP production, treatment and post-treatment rehabilitation protocols.6,12,23–25
Prolotherapy is an additional therapy that introduces a small amount of a natural irritant solution to the site of painful and degenerated tendon insertions, joints, ligaments, and adjacent joint spaces.26 Commonly, prolotherapy consists of a concentrated dextrose solution that creates an environment to reintroduce a local inflammatory cascade, triggering the release of growth factors that aid with collagen deposition around areas of injury or laxity.12,26,27 Hence, a major goal of prolotherapy is to treat ligamentous laxity and related musculoskeletal and arthritic conditions by improving the tensile strength of specific intra-articular and extra-articular tissues.12,26,28 Overall, the evidence supports the use of dextrose prolotherapy for tendinopathy, specific joint osteoarthritis, and spinal/pelvic ligament dysfunction.26 A recent trial29 reported short-term (2-week) improvements in pain, disability and shoulder AROM following prolotherapy to patients with supraspinatus tendinopathy. Smaller studies have given some plausible insight for the use of prolotherapy for the ACJ and complex shoulder injuries, however, there are no larger trials to reinforce these findings.30,31
Rehabilitation following orthobiologic therapy is described by the American Physical Therapy Association as the integration of interventional orthobiologic techniques coupled with appropriate rehabilitation strategies that harness the healing mechanisms through movements to augment the orthobiologic therapies.12 The goal of rehabilitation after orthobiologic therapy is to return a patient to optimal function by combining the science and research behind both rehabilitation and regenerative medicine. Further preliminary data with progressive tissue healing protocols are needed to support its use following orthobiologic therapy.
In this case, four unique methods were incorporated to optimize this patient’s healing and functional recovery. These included blood flow restriction therapy (BFR)32–35 prior to the blood draw (pre-exsanguination) and during specific rehabilitation, dry needling with electrostimulation,36–38 and a specific protein supplement regimen designed to amplify the effects of tissue loading.39,40 Each of these unique elements were combined in a manner not before described in the literature. The purpose of this case report is to describe the distinct method including orthobiologic preparation, tissue-specific treatment and regenerative rehabilitation of an athlete with a complex shoulder injury.
CASE DESCRIPTION
A 15-year-old competitive female wrestler with persistent shoulder pain presented after unsuccessful management of her shoulder pain with conventional physical therapy. The injury occurred during a wrestling practice when another wrestler fell (full bodyweight) on the anterior shoulder in a position of end range abduction/external rotation. No immediate diagnosis was made on the field. The athlete was able to continue with wrestling but with significant limitations in her performance, persistent resting pain and disturbed sleep. Following the wrestling season, she underwent three months of physical therapy including glenohumeral joint mobilizations, range of motion and traditional rotator cuff exercises. This rehabilitation approach resulted in minimal improvement in symptoms or sport specific function. The athlete had no prior injuries to the shoulder, cervical, or thoracic spines.
The subject subsequently presented to the physical therapist and physician for a co-evaluation, The physician was a dual-board certified anesthesiologist and pain management physician with a specialty in regenerative medicine. The physical therapist was board certified, and fellowship trained in orthopaedics. Informed consent was obtained from the subject/parents prior to the history and physical examination. The subject’s pain on the numeric pain rating scale (NPRS)41,42 was 5/10 at rest and 8/10 during athletic activity (Figure 1). She described the pain as widespread with poor localization, and her initial Quick-DASH43,44 score was 18% (Figure 2). The subject was unable to perform wrestling off-season practice or run due to a feeling of instability and pain in the shoulder. Pull-up motions and bench press motions were notably limited due to pain and feelings of instability during supervised team weight training. Further. skills position of a wrestling “under-hook” position could not be performed due to instability. Importantly, prior to injury, her spring sport participation was unrestricted and included four days per week of wrestling practice with three days per week of strength training. Pre-injury, one-repetition maximum testing was reported to be 65lb bench press, 50lb pound dumbbell row, and 145lb deadlift.
PHYSICAL EXAMINATION AND DIAGNOSTIC IMAGING
Physical Examination
Visual observation revealed normal position of the glenohumeral joint (GHJ) with a minor misalignment/swelling around the ACJ. Initial screening for the cervical spine involvement was negative and included a negative Spurling’s test for reproduction of symptoms, a positive Shoulder Abduction Sign for exacerbation of shoulder symptoms, and no symptoms below the elbow.45 The initial shoulder exam revealed full active range of motion in flexion, abduction, external rotation at 90 degrees abduction and internal rotation at 90 degrees abduction. Pain was reproduced from 90 degrees of shoulder elevation through end range, with more severe pain at end range. Impingement testing was negative.10,46 Special testing for the labrum revealed a positive compression rotation test, with pain reported deep in shoulder.47,48
Special testing for the acromioclavicular joint included painful palpation, cross-body adduction, resisted acromioclavicular extension, and a positive active compression test.49,50 Laxity was in the ACJ joint in anterior to posterior direction. Superior glide mobility testing of the ACJ was painful, with a firm ligamentous end-feel when compared to the contralateral side. Laxity of the GHJ was also noted ipsilaterally during the anterior load and shift test.51
Strength of the rotator cuff (internal and external rotators) was 5/5 in neutral position but was limited by pain (4/5) in 90 degrees of flexion and abduction. Strength testing of the scapular musculature (middle trapezius, lower trapezius, rhomboids) in prone was similar in that testing at greater than 90 degrees of shoulder flexion or abduction was limited by pain. The tentative diagnosis from the physical examination included an ACJ sprain and suspected labral tear with anterior laxity of the glenohumeral joint.
Diagnostic Imaging
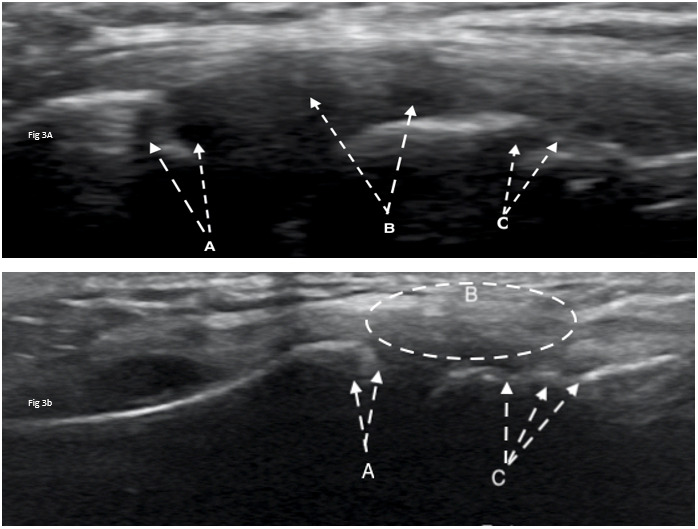
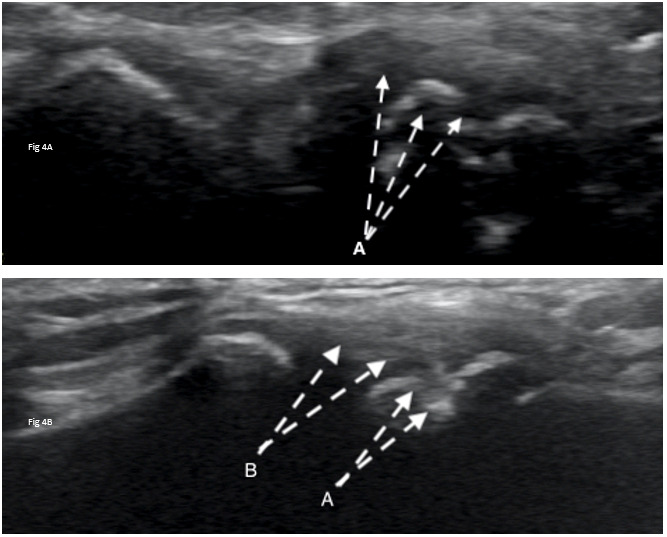
Diagnostic ultrasound imaging (GE Logiq E9; 2010) of the biceps tendons, tendons of the rotator cuff, rotator interval, labrum and anterior/posterior ACJ was performed immediately following the physical examination. Ultrasound confirmed misalignment of ACJ (clavicular portion elevated) with distinct hypoechoic patterns noted on both the clavicular and acromial attachments of the capsule, with associated bony abnormalities (Figure 3A, 4A). Further, a small supraspinatus myotendinous partial thickness tear was also identified. The labrum was not adequately visualized with the ultrasound study.
Based on the physical examination/ultrasound findings and a lack of progress with conservative treatment, magnetic resonance imaging (MRI) was ordered to guide further treatment considerations. An MRI with contrast was performed using a 3-tesla magnet and revealed a small focal tear in the superior labrum at the biceps labrum attachment (Figure 5A), with an intact biceps tendon. Further, arthritic/cystic changes were noted in the acromion, though the ACJ ligaments were still intact. Importantly, the supraspinatus tear found during the diagnostic ultrasound was not adequately visualized on the MRI scan and the labral tear found on the MRI was not adequately visualized on the ultrasound. Hence, both imaging techniques were indicated.
INTERVENTIONS
Due to the complex nature of the shoulder injury (ACJ, labrum, rotator cuff) and the athlete having an impending soccer season, the labrum was prioritized, and addressed first with a PRP injection. The PRP procedure was the initial focus, because of the inflammatory cycle52 and the longer period of recovery needed with this type of regenerative procedure.18,20,21
Pre-PRP Preparation
Prior to exsanguination, a novel technique of blood flow restriction (BFR) exercise53 was performed (Appendix) to amplify important circulatory components of healing in in the subject’s whole blood.16,32,33,35,54–56
PRP Preparation
The platelet-rich plasma was prepared using 160mL whole blood exsanguinated using a 20-gauge antecubital access. This was drawn into 10mL vials containing citrate as an anti-coagulant. The first spin was performed at 1500 RPM for 10 minutes to segregate the plasma, platelets, and leukocytes from red blood cells. The serum layer was extracted manually down to buffy coat and transferred to two new vials. The second spin was performed at 2500 RPM for eight minutes. The serum was removed from each vial until 6mL of serum with a platelet plug remained from each vial. This was then mixed to reconstitute a total of 12mL of leukocyte rich platelet-rich plasma (LR-PRP), which is approximately 13x the concentration of platelets.
Ultrasound Guided LR-PRP Injection
The injection area was identified with Logiq E9 ultrasound using a linear probe in virtual curvilinear mode. To target the biceps tendon insertion on the labrum and the labral tear, the probe was positioned in the anterior shoulder in an axial plane to visualize the pectoralis major and short head of the biceps tendon overlying the anterior labrum. The needle was inserted in short axis and advanced to the labrum, confirming needle placement with hydro-dissection of tissue using LR-PRP. The needle was progressively walked cephalad noting the needle palpatory feedback and ultrasound visualized hydro-dissection to confirm placement. Upon overlap with the coracoid process and loss of visualization of the labrum, the needle was directed cephalad by palpation alone. Approximately 4mL of LR-PRP was injected into the labrum and intra-articular space. Despite its absence on MRI, a small myotendinous junction supraspinatus tear was appreciated on ultrasound, and the remaining 2mL of LR-PRP was injected into the supraspinatus myotendinous junction and sub-acromial bursa.
The subject was followed regularly with a specific physical therapy protocol based on time frames of recovery to aid with tissue loading and healing.52 This protocol was developed in order to better stimulate growth factors15,16,53 and encourage stress relaxation of the collagen associated with the injured tissue.39 Prior to each exercise session the subject was instructed to consume 15 grams gelatin in the form of hydrolyzed collagen combined with 225 mg vitamin C to help stimulate collagen synthesis, which in turn has the potential to improve the function and integrity of an impaired tendon.39,40,57 In order to further enhance the healing response, the subject received electrical dry needling38 to the shoulder according to a protocol developed by Dunning et al.10 All components of the post-procedural rehabilitation program are detailed in the Appendix.
OUTCOMES
Following successful completion of her eight-week plan of care after the PRP injection, the subject noted a distinct difference in the level and location of pain. Prior to the PRP intervention, she was unable to localize pain in her shoulder and described it as wide-spread pain at rest (NPRS=5/10) and with movement (NPRS=8/10). After the PRP and regenerative rehabilitation, she noted the lower/inferior 50% of her shoulder pain had resolved but an upper, more anterior pain remained at rest (NPRS=2/10) and with movement (NPRS=5/10). This was correlated clinically with negative special testing of the labrum/rotator cuff and no changes in the provocative ACJ exam. These findings from the follow-up examination and self-report outcomes (Figure 1, 2) helped confirm the labrum tissue as a potential independent source of pain, allowing a more confident transition into phase II of her orthobiologic intervention.
The second phase began with an ultrasound guided prolotherapy injection (20% dextrose + 0.2% lidocaine) into the anterior acromioclavicular joint. A second prolotherapy injection was determined to be necessary at a follow-up visit due to posterior ACJ pain during heavier weightlifting and plyometric activities. The second ultrasound guided prolotherapy injection (with identical concentration) was directed to the posterior ACJ based on local tenderness and symptom provocation. A less rigid rehabilitation protocol was followed in this phase due to the nature of healing with prolotherapy.58 (Appendix).
At the three-month (13 week) follow-up after the initial treatment, disability dropped to a 0%, and the subject was able to perform soccer, heavy weightlifting, and intense wrestling practice without limitation or pain (Figure 1, 2). Reevaluation of provocative special testing of the shoulder at three months was negative. Intense wrestling practice at the three-month time frame included plyometric activities through the shoulder, ability to perform under-hook positions dynamically and against resistance, and full painless participation in supervised team weight training. Weight training returned to pre-injury strength testing levels and subject returned to running without feelings of instability or pain, allowing unrestricted participation in soccer and wrestling concurrently.
At the one-year follow-up, the subject reported no pain (NPRS=0/10) and full return to athletics (QDASH =0% disability). A follow-up MRI at one year revealed no discrete labral tears, minimal supraspinatus tendinopathy, and normal visualization of the ACJ (Figure 5B). Similarly, a one-year follow-up diagnostic ultrasound revealed normal appearance of the supraspinatus tendon, its myotendinous junction and ACJ (Figure 3B, 4B).
DISCUSSION
This case report describes the successful short and long-term outcome of a competitive female wrestler with a complex shoulder injury who failed conservative treatment and returned to sport without pain or the need for surgery. The novel methods of PRP preparation, tissue specific orthobiologic therapy, and regenerative rehabilitation protocols may have contributed to the overall outcomes and timely return to full function.
The use of PRP for tissue healing is based on the underlying capacity of platelets to supply and release supraphysiologic amounts of essential growth factors and cytokines from their alpha granules. This process provides a regenerative stimulus that augments healing and promotes repair in tissues with low healing potential.13 Growth factors that are commonly described for their utilization include but are not-limited to: platelet-derived growth factor (PDGF), transforming growth factor-b (TGF-b), vascular endothelial growth factor (VEGF) epidermal growth factor (EGF), basic fibroblast growth factor (bFGF), and insulin-like growth factor (IGF-1).13,59
Blood flow restriction (BFR) exercise has been described as a training method which partially restricts arterial inflow and fully restricts venous outflow in the working musculature during exercise.34 It exerts physiologic effects under low loads that would normally occur under heavier loads but cannot be performed due to injury, healing or surgery. This has been shown to produce improved strength and muscle hypertrophy changes in a more expedient manner than with traditional high-load strength training.60,61 It additionally improves a variety of measures correlated with improvements in strength and the positive effects of exercise.35,62,63 It has been suggested that BFR exercise results in increased mobilization of hematopoietic stem progenitor cells, proliferation of myogenic stem cells, increased cytokine responses, increases in the number of circulating CD34+/KDR+ endothelial progenitor cells, and increased VEGF.32,33,35,54–56 Thus, in this case report, BFR was used during exercise to “amplify” the yield / healing capacity of PRP by utilization pre-exsanguination and during rehabilitation, respectively. A recent study suggested that BFR can be considered a way to manipulate point-of-care blood products such as PRP to increase product yield.16 To date, no prior studies have used BFR as an adjunct to orthobiologic therapy or as a part of the regenerative rehabilitation process.
Dry needling with electrical stimulation was incorporated after exercise to continue to facilitate the healing process after orthobiologic injection. Dry needling is described as an intervention whereby thin, monofilament needles are used to penetrate the skin and stimulate underlying neural, muscular and/or connective tissues for the management of pain and disability associated with neuromusculoskeletal conditions.64 Dry needling with electrical stimulation has been found to aid with improved analgesia in many painful conditions, including subacromial pain syndrome, and has been shown to aid with wound and tissue healing in experimentally induced trauma.37,38,65–69
To further attempt to augment the healing process, the subject was required to take 15 grams of hydrolyzed collagen with 225 mg of vitamin C one hour prior to rehabilitation exercise. This was consistent with prior research supporting collagen synthesis and tendon healing.39,40 Although Baar39 described the successful rehabilitation of patellar tendinopathy, it appears that tendons and the shoulder labrum both contain a similar collagen matrix,70 and may result in similar changes when supplementing the effects of the regenerative rehabilitation protocol.39,40,57,71,72 Further research is needed in this area.
Following, the first phase of the orthobiologic/rehabilitation process, prolotherapy was directed at the ACJ to reduce laxity and address remaining pain in the shoulder. Interestingly, there were greater reductions in disability after the prolotherapy injections (16% to 0%) than the PRP injections (18% to 16%). Similarly, activity related pain (pain at worst) followed a similar trend after the PRP (8/10 to 5/10) and prolotherapy (5/10 to 0/10). Clinically and pathoanatomically there may be several plausible theories behind these changes. First, with multiple areas of tissue injury, it is very difficult a patient or the clinician to decipher individual components of injury and related pain/disability. Second, the reduction in the initial higher pain thresholds after the PRP/rehabilitation may have been related to the selected tissue healing and initial return to function. However, the laxity and remaining pain in the ACJ tissues during the provocative exam may have been the key pathoanatomical component associated with the stagnant disability. From an orthobiologic/regenerative medicine perspective, addressing the correct tissue based on its healing time frame, surrounding structural integrity, and results of imaging and physical examination seems to be of paramount importance.
Finally, it should be noted that because this is a case report, the results or theoretical constructs do not indicate a cause-and-effect relationship. Results such as those experienced by this subject may not be seen in all subjects. Additionally, a six-month follow-up with diagnostic ultrasound and or MRI would have been ideal to correlate with specific tissue healing. Further investigation comparing outcomes after conservative versus typical surgical management is needed. Additionally, future studies/trials need to consider standardizing blood-based orthobiologic preparation and specific regenerative rehabilitation protocols to better determine the effect of these novel techniques.
CONCLUSION
This case report demonstrates the successful outcome of a competitive female athlete with a complex shoulder injury who failed prior conservative rehabilitation. Detailed imaging and evaluation procedures indicated tissue pathology at multiple joint sites. Tissue specific orthobiologic interventions with novel preparation, in conjunction with a comprehensive regenerative rehabilitation protocol, resulted in a full return sport without restrictions at the three-month follow-up. At the one-year follow-up, MRI and diagnostic ultrasound imaging confirmed notable tissue healing in all original injury sites.
Conflicts of Interest
The authors report no conflicts of interest.
_1wk-7wk_(outcom.png)




_1wk-7wk_(outcom.png)