INTRODUCTION
Plantar fasciitis (PF) is the leading cause of heel pain in ambulatory settings, affecting up to 10% of adults.1–3 Though the name is misleading, PF is not primarily an inflammatory condition.4,5 Repetitive trauma to the connective tissue causes acute inflammation. However, it is the combination of tissue destruction, fascial thickening, collagen necrosis, matrix calcification, peri-fascial edema, and alterations in vascularization that lead to the debilitating pain associated with PF.4,6–9
Conservative PF treatment (e.g., reduced activity/loading, icing, stretching, orthotics, and taping/bracing) typically spans 6-12 months, and improvements are not often seen before six weeks of therapy.1,2,10 In some resistant cases, more aggressive, sometimes painful and invasive treatments are required, such as corticosteroid injections, radiation,11 platelet-rich plasma injections,6 and surgery.1,2,12
Photobiomodulation (PBM) is an emerging therapy that uses non-ionizing, visible and near-infrared light to affect endogenous chromophores and elicit photochemical events at the cellular level.13 PBM therapy (PBMT) has been shown to improve other tendinopathies in studies of lateral epicondylitis, shoulder tendinopathy and Achilles tendinopathy when using optimized wavelengths and dosing parameters.14–16 The clinical benefit of PBMT for tendinopathies is thought to be mediated by collagen production,17 alignment of collagen fibers,18 and other mechanisms.19
Recent meta-analyses have reported positive findings supporting PBMT as an effective treatment modality for PF, though the conclusions are somewhat heterogenous due to inconsistent dosing parameters (e.g., wavelength, power, application duration, intensity) and study methodologies.20,21 Specifically, the “dose” of PBMT is denoted by the intensity (J/cm2) of the light delivered to the target area. However, the intensity is a product of the power (W) and application duration (sec); thus, equivalent “doses” could be achieved by proportionately increasing or decreasing both the power and application duration. Unfortunately, many studies do not adequately report these values, making direct comparisons difficult. As with any treatment, choosing the correct dose is essential to optimizing safety and efficacy.22 Of the multiple parameters for PBMT, wavelength and power are likely the most important, as wavelength determines the depth of photon penetration and power determines the number of photons delivered to the target tissue. PBM in the 810-980 nm wavelength range is known to penetrate the skin and superficial tissues to reach underlying tissues, such as muscle and tendon, including the target tissue of the plantar fascia.23
PBMT is non-invasive and has potential to address the root cause/dysfunction of the injury, decrease the pain of PF quickly, and return individuals to increased function and physical activity. The goal of this study was to assess the clinical impact of PBMT on pain and function in people with PF.
MATERIALS AND METHODS
Trial Design & Participants
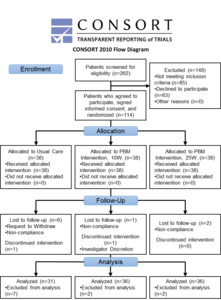
This prospective, randomized controlled trial was conducted at a United States military medical center in Germany. Recruitment and enrollment of participants (n = 114) targeted adults between 18-65 years of age with symptoms of PF for at least three months (diagnosed by their primary healthcare providers, e.g., MD, DO, PA, NP), able to read and understand English language for consent purposes, and able to commit to six-week intervention and three and six month follow-up. Candidates were excluded for having a history of trauma, fracture, previous corticosteroid injections or other invasive treatment for PF to the symptomatic foot. Candidates with neuropathy or altered detection of skin temperature were excluded (including use of medication that may lead to the same), as well as those with greater than 15% of calf covered in tattoos, since pigment in ink absorbs light and can cause overheating of skin. Additionally, pregnant females and candidates with pacemakers were excluded.
The study protocol conforms to the Declaration of Helsinki and was approved by the Institutional Review Board of record (M-10548) and registered at ClinicalTrials.gov (registration number NCT03015116). Informed consent was obtained from all participants prior to enrollment in the study. Upon study enrollment, patients were allocated to study groups by the principal investigator, ensuring no healthcare provider bias. A parallel assignment study intervention model was employed. Due to the nature of the intervention, healthcare provider and patient blinding was not plausible, thus this study was conducted as open label.
Interventions
Usual Care Protocol
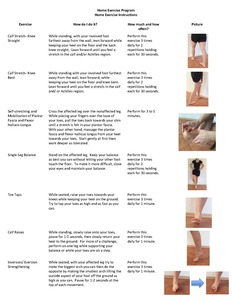
All participants were instructed to complete a usual care (UC) protocol daily for six weeks, beginning on day one, based on recommendations by current Clinical Practice Guidelines for PF treatment (Figure 1).24–26 The UC group participants were given the opportunity to receive PBMT outside of the study protocol for their affected foot after the completion of the 6-week study period.
PBMT Protocol
Both intervention groups received PBMT three times a week for three weeks for a total of nine treatments and completed the UC protocol daily for six weeks, beginning on day one. All participants in both PBMT groups received the same dose at each treatment session. The only difference between groups was whether PBMT was delivered fast or slow. Here, “fast” PBMT refers to a dose of 10 J/cm2 delivered for one second per square centimeter of skin at 10W, while “slow” PBMT refers to the same dose of 10 J/cm2 but it was delivered for 0.4 seconds per square centimeter of skin at 25W.
To achieve a standardized PBMT dose of 10 J/cm2, the study team calculated the area of each participant’s foot and calf at baseline and varied the time over which the total dose was delivered by calibrating to the output power (10W or 25W). Providers used a diode laser (LightForce EXPi, LiteCure/LightForce Medical, New Castle, DE, USA), with a blend of 20% 810nm and 80% 980 nm wavelength, continuous wave light delivered via a hand piece with an approximately 7 cm massage ball. Participants lay in a prone position, and the provider treated the plantar foot and dorsal calf surfaces in a serpentine movement, with the massage ball in perpendicular contact with the skin, slightly compressing underlying tissues. Treatment time was split equally between the foot and the calf, with intermittent passive range of motion of the ankle.
Outcomes
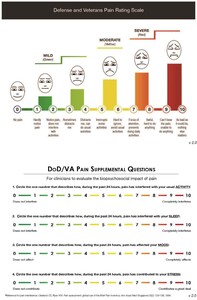
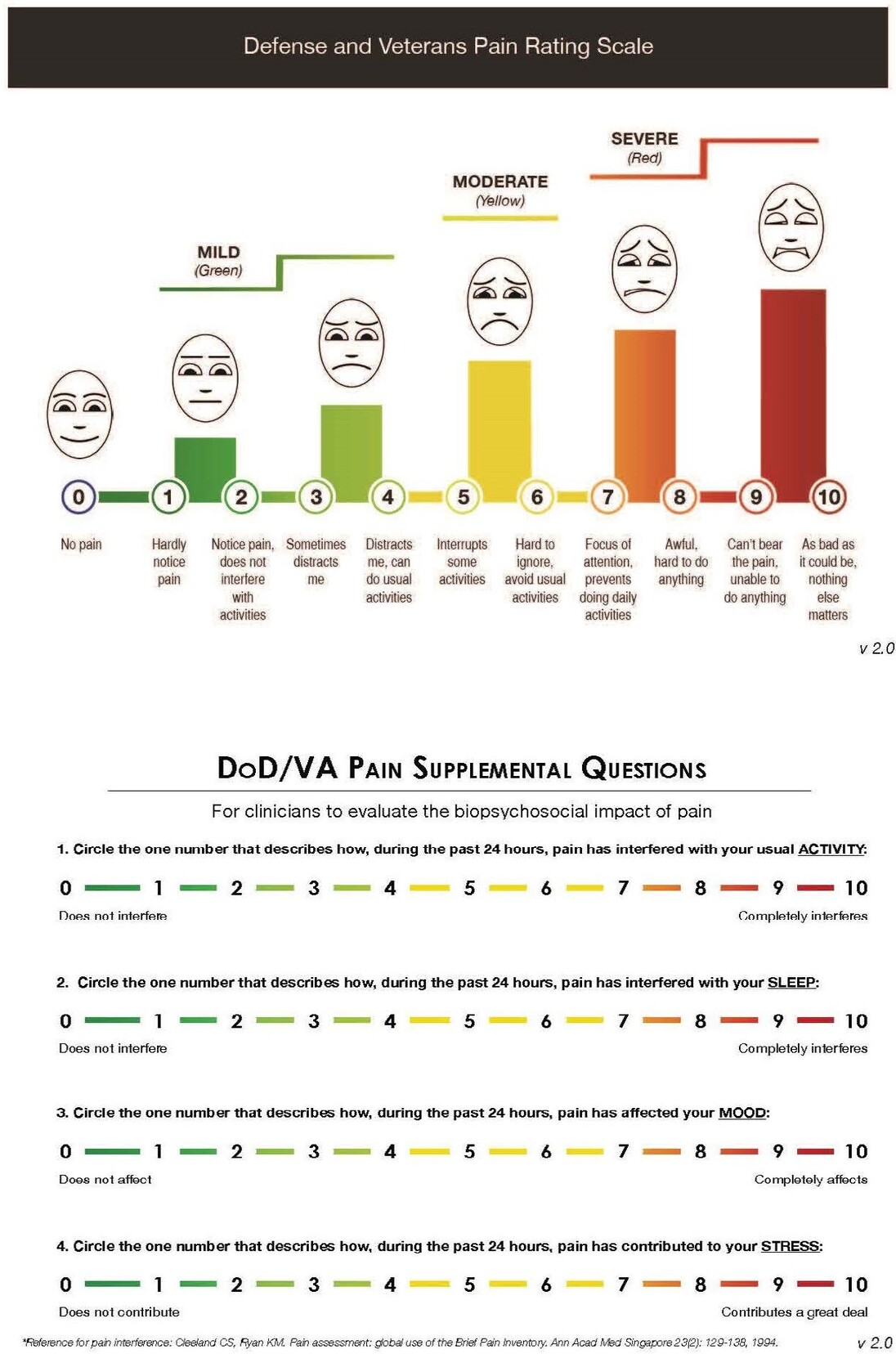
The primary outcomes were pain, assessed by the Defense and Veterans Pain Rating Scale (DVPRS),27–29 and function, assessed by the Foot and Ankle Ability Measure (FAAM).30 The 5-item DVPRS integrates a numeric pain rating scale with visual facial cues and word descriptors and four supplemental questions measuring pain interference (Figure 2).27,28 Permission is granted for clinicians and researchers to freely use the DVPRS as is, without alteration. All participants completed a daily DVPRS diary for six weeks, beginning on day one. In addition, four DVPRS supplemental outcomes were tracked: self-reported activity, mood, sleep interference, and stress.
The FAAM is a 29-item self-report instrument that assesses physical function in foot and ankle impairments. There are two subscales: activities of daily living (ADL) (21-item) and sports (8-item).The subscale items are scored on a 5-point Likert scale (4=‘no difficulty at all’ to 0=‘unable to do’) and then the points are converted to a percentage (100%=no dysfunction).30 To measure the long-term outcomes in the PMBT groups, the study team sent a password-protected fillable PDF file of the DVPRS and FAAM to the PBMT participants via email so participants could report their pain and function at 13 and 26 weeks.
Participants also completed a daily medication and activity diary for descriptive analysis.
Sample Size
Glaser Consulting performed power analysis using G*Power software.31 Considering the exploratory nature of the study and estimated population of participants, a small to medium effect size of 0.15 and autocorrelation of 0.3 was chosen, requiring n = 96 participants, or n = 32 participants per group. Participants were over-recruited by 20% (n = ~114, or n = 38 participants per group) to account for the potential attrition.
Randomization
An Excel random number generator was used to assign participants to UC, UC plus 10W PBMT, or UC plus 25W PBMT, yielding 38 participants in each group.
Statistical Methods
Descriptive statics reported distributions of baseline demographics, daily activity and medication diary data. Inferential statistics used a nonlinear extension of generalized linear models, Hierarchical generalized additive models (GAM) using patient-level random effects with Gaussian distribution families. The hierarchical modeling with patient-level random effects handles the correlation of within-patient repeated measures, and the nonlinear GAM captures nonlinear effects.32 Where appropriate, hypothesis tests were two-sided and considered significant at the putative threshold (alpha=0.05). Due to their nature, nonlinear effects are not interpreted in terms of coefficient p-values. Only linear effects have coefficient p-values that are readily interpretable. Nonlinear effects are interpreted graphically—that is, by inspecting partial dependence plots. These partial dependence plots are intuitive because they display the average patient-level effect in terms of its mean and confidence band. To assess whether effects are different between groups, simply look at their partial dependence plots; where the groups’ confidence bands overlap, their effects are not statistically different; and where their confidence bands do not overlap, the effects are statistically different. In this way, partial dependence plots are intuitive and carry more information than coefficients and p-values from linear models. In other words, a modeling approach is used that is interpreted by looking at graphs, not p-values. Data normality and homoscedasticity were verified by Kolmogorov-Smirnov and Breusch-Pagan tests prior to analysis, respectively.
RESULTS
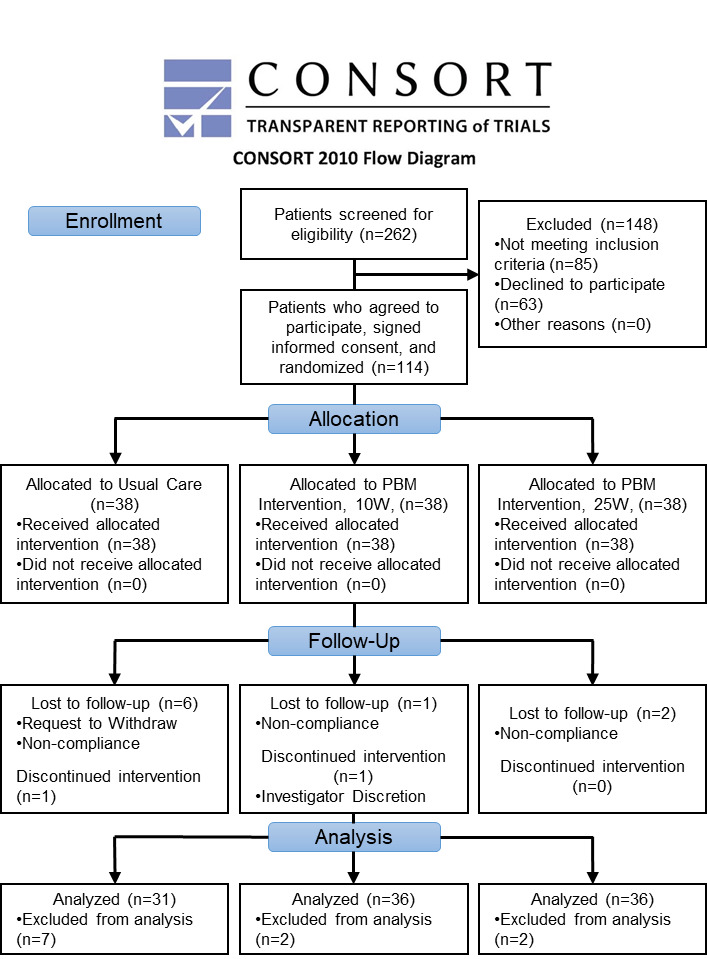
Participant Flow and Numbers Analyzed
By the end of the initial six weeks, seven, two and two participants had withdrawn from the UC, 10W, and 25W group respectively, leaving 31, 36, and 36 participants in each group with complete data for analysis of primary and secondary outcomes. UC group participation in the study ended after six weeks. For long-term follow-up, 88% of the PBMT groups were retained at three months and 76% by six months (Figure 3).
Baseline Data
Baseline demographic data showed representation among males (43.8%) versus females (56.3%); among Caucasian (58.8%) versus non-Caucasian (38.7%); and among military (47.4%) versus non-military (52.7%) (Supplemental File 1). The mean age of the participant population was 43.4 years old. While the study population showed a high percentage of overweight and obese participants based on self-reported data, there was no significant difference between groups.
Outcomes and Estimation
Pain
Because there was no difference in outcomes between the two treatment groups, for the primary analysis, the PBMT groups were pooled. Both PBMT groups received the same dose (10 J/cm2), with the only difference being the “speed” at which the total dose was delivered: either “slow” at one second per square centimeter, or “fast” at 0.4 seconds per square centimeter.
The pooled PBMT groups experienced a reduction in pain over the first three weeks, with a patient-level average change from 4.47 + 0.13 to 2.84 + 0.07. The UC group experienced a small reduction in pain over the same period, having a patient-level average change from 4.03 + 0.15 to 3.76 + 0.08. The effects on pain were not meaningfully different between PBMT groups, which were assessed graphically (Figure 4). Recall, the statistical significance of non-linear effects are interpreted not by coefficient estimates but by comparing confidence bands in partial dependence plots.
From three to six weeks, pain reduction appeared to plateau in the pooled PBMT groups (six-week mean 2.70, SE 0.1) and in the UC group (six-week mean 3.70, SE 0.15, n=24). When stratifying the 10W and 25W PBMT groups apart, no significant difference was observed, which is illustrated by their confidence bands (2*SE) overlapping in the partial dependence plot (Figure 4).
Function
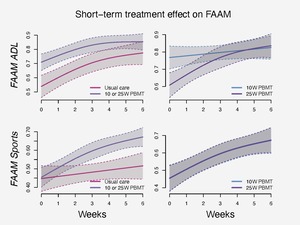
Functional outcomes also showed some improvements in PBMT groups compared to UC (Figure 5). Patient-level average function per the FAAM sports subscale improved in the pooled PBMT groups from baseline (mean 0.45, SE 0.03, n=73) to six weeks (mean 0.66, SE 0.03, n=70), while functional improvement was slight, at best, in the UC group from baseline (mean 0.45, SE 0.04, n=37) to six weeks (mean 0.50, SE 0.04, n=32). There was no measured difference between the 10W and 25W PBMT groups in terms of FAAM sports.
Function, per the FAAM ADL subscale, did not seem to improve more in the pooled PBMT groups from baseline (mean 0.71, SE 0.02, n=76) to six weeks (mean 0.82, SE 0.01, n=70) than it improved in the UC group from baseline (mean 0.57, SE 0.02, n=38) to six weeks (mean 0.75, SE 0.03, n=32).
In contrast to the UC group, individuals in both PBMT groups reported notable (but non-significant) enhancements in the FAAM ADL subscale, surpassing validated thresholds for clinically meaningful changes at both 6-week and 6-month intervals. PBMT group participants met both the Minimal Detectable Change and Minimal Clinical Important Difference cutoff change scores of 6 and 8, respectively, when calculated at both six weeks and six months after treatment. These findings indicate that there is reasonable certainty of true change (95% CI) and that the change is clinically meaningful to the participants.30
Ancillary Analyses
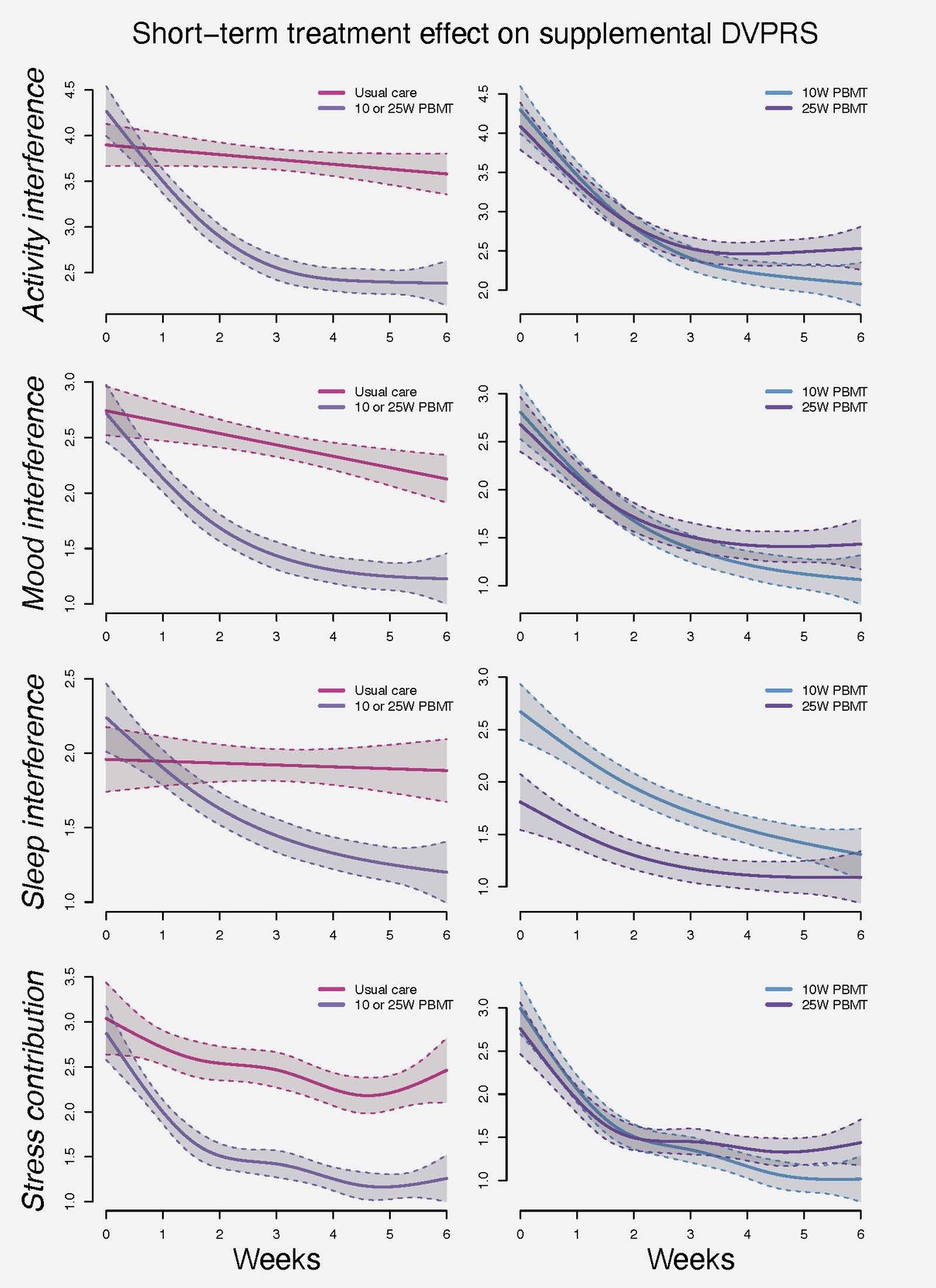
Supplemental DVPRS
The pooled PBMT groups demonstrated improved average patient-level changes to activity interference (from baseline 4.4 (SE 0.1) to six weeks 2.3 (SE 0.1)) compared to UC (from baseline 3.9 (SE 0.1) to six weeks 3.6 (SE 0.1)). Similar trends were observed for mood interference, sleep interference and stress contribution (Figure 6).
Fitzpatrick skin type subgroups
Fitzpatrick Skin Type was significant in both the FAAM sports (b= -.05, p = .03) and FAAM ADL (b = -.04, p = .002) subscales, with the negative coefficient indicating a higher Skin Type score was associated with a lower FAAM score. Findings for pain outcomes were not significant between Fitzpatrick categories.
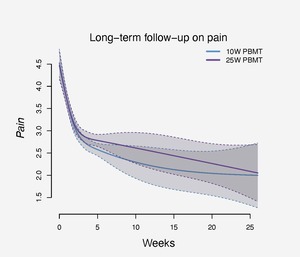
Long-term Follow-up
Participants in both PBMT groups reported stable pain and function outcomes at 13 and 26 week follow-up time points. (Figures 7-9)
DISCUSSION
There were three main findings as relates to the study’s primary aims: 1) PBM therapy at both power levels (i.e., 10W and 25W; both administered to achieve 10J/cm2 dose) resulted in clinically relevant significant reductions in pain, whereas the UC group did not exhibit reductions in pain, 2) PBM therapy at both power levels resulted in some increases in the FAAM Sports subscale; however, this did not achieve the level of statistical significance and no differences were noted in FAAM ADL between both PBM groups and the UC group, 3) no statistically significant differences were noted in pain, FAAM Sports, or FAAM ADL between the 10W and 25W PBM groups.
Two recent systematic reviews and meta-analyses reported significant improvements in pain (Visual Analog Scale) and function (Foot Function Index) in favor of PBMT over control.22,33 Other PMBT studies for PF, including those in recent meta-analyses, used different outcome measures, wavelengths, and other parameters, and often did not report their methods completely making direct comparison challenging.20,21,34–38 However, the findings are consistent in that pain and function are improved over time when the appropriate wavelength and other treatment parameters are chosen (i.e., power, application time).
Participants in this study reported a mean baseline pain level between 4.1-4.3, and the UC group reported this consistent level of pain through the end of the protocol. In contrast, participants in both PBM groups reported clinically relevant and significant decreases in pain throughout the 6-week protocol period. Additionally, both PBM group participants reported a two-point decrease in the pain scale by long-term follow-up. While long-term data were not collected for the UC group, their consistent pain scores through the study period stand in stark contrast to the decreases noted in the PBM groups. Salaffi and colleagues analyzed clinically meaningful change in numeric pain rating scale scores in chronic musculoskeletal injuries and reported that patients considered their pain to be “much better” when their scores decreased by 2 points.39 This cutoff was reached in both PBMT groups at their long-term follow-up demonstrating clinical improvements in pain and long-term relief of PF symptoms. While it was not a direct aim of this study, medication diary descriptive statistics indicated that daily non-steroidal anti-inflammatory drug consumption decreased in the treatment groups but remained steady or increased in UC participants. This is an interesting finding that warrants further study considering the risks of long-term non-steroidal anti-inflammatory drug use.
While this study did not explore the underlying biological mechanisms for decreased pain in the PBM groups, other works have investigated the impact of PBM on various tendinopathies and may inform the results seen herein. It is well known that effective treatments for PF and other tendinopathies must address the underlying injury mechanism versus the symptoms only. Chronic PF (and other tendinopathies) results in a recurring cycle of degeneration.4 Animal studies using PBMT in other tendinopathy models support that correctly dosed PBMT penetrates to the fascia and results in beneficial changes to the degenerated tissue. These changes include synthesis, organization, and strengthening of damaged collagen fibers, activation of matrix metalloproteinases, cellular proliferation and new blood supply growth.19,40–42 In clinical trials, investigators have reported significant decreases in plantar fascial thickness, indicating restructuring of damaged fascia.35,38 Taken together, these findings provide support to explain the self-reported improvement in functional outcomes in this and other studies, and future studies should include these objective measures of structural change.
Selection of wavelength and other treatment parameters is essential for effective treatment. The vast majority of PBMT studies in PF select wavelengths in the near-infrared range. At lower wavelengths (500nm to 800nm), melanin in the skin is the primary chromophore, limiting penetration to deeper structures, however light in the 800nm to 1000nm range is ideal for reaching the injured tissue in PF.23,43
Because study patients were treated over both the plantar surface of the foot, which typically has less melanin than other skin, as well as the dorsal calf and ankle, the impact of Fitzpatrick skin type on outcomes was evaluated. The study had a significant finding that higher Fitzpatrick category was predictive for poorer outcomes in the FAAM ADL and sports subscale, but had no significant impact on pain. Though these results should be interpreted cautiously due to the small number of participants in higher Fitzpatrick categories, it is important to remember when designing treatment protocols to consider using a longer wavelength option (e.g., 980 nm) for those individuals.
Implications
With advances in technology making devices with higher power outputs available, understanding appropriate use of PBMT parameters is more essential than ever before. The current study utilized a simple area equation to ensure all participants received a standardized energy density of 10 J/cm2, regardless of power output. Participants in the both the 10W and 25W groups tolerated the treatment well with no adverse outcomes related to the treatment and reported similar improvements in pain and function. The primary difference between the treatment groups was in the time required to complete the treatment – given the same surface area treated, using 25W is 2.5 times quicker than 10W. In a clinical setting, this equates to being able to treat more patients safely and effectively, while reserving the option to decrease the power output at their discretion without jeopardizing patient outcomes.
Limitations
The positive outcomes of this study are limited by the absence of a sham treatment group; however, at the time of the study, an indistinguishable sham control option was not available.44 Secondly, the lack of long-term follow-up in the UC group does limit the utility of the long-term data from both PBM groups. Finally, though conducted in a military treatment facility setting, the demographic distribution of participants are consistent with similar trials,22 and therefore, supports this treatment protocol for future studies and clinical treatment. Future studies should investigate the impact of even higher-powered lasers (e.g., 40W) to ensure the lack of difference between power groups reported herein persists with even higher laser powers.
CONCLUSION
The standardized PBMT protocol plus UC resulted in statistically and clinically significant decreased pain and improvements in function in the FAAM sports subscale compared to usual care alone. Additionally, there was no difference in outcomes between the groups receiving 10W and 25W output power with a standardized energy density of 10 J/cm2 over the long-term, with improvements being sustained at the 6-month follow-up point. The lack of adverse events and significantly and clinically meaningful decreases in pain scores support previous work that PBMT is a safe, innovative treatment targeting the root cause of injury to the PF.
Acknowledgements
TriService Nursing Research Program (N16-P09) provided the funding to conduct this study, and the device was provided via a Cooperative Research and Development Agreement between LiteCure, LLC and the Clinical Investigation Regulatory Office, U.S. Army Medical Research and Materiel Command.
Ann Ketz, RN, PhD is currently employed by Enovis, DJO Global (now Enovis), who acquired LiteCure, LLC; she was not employed by them for the duration of the study. Juanita Anders, BA, MS, PhD, has received Cooperative Research and Development Agreement (CRADA) between USUHS and Lite Cure, LLC; received equipment from B&W Tek, Irradia, Lite Cure, Nitto Denko, PhotoThera; serves on advisory board for Lite Cure, LLC. The remaining authors certify that they have no affiliations with or financial involvement in any organization or entity with a direct financial interest in the subject matter or materials discussed in the article.
The study was registered at ClinicalTrials.gov (registration number NCT03015116) and was carried out in accordance with ICH/GCP guidelines.
Disclaimer
The information, content, and conclusions in this paper do not represent the official position or policy of, nor should any official endorsement be inferred by, the TriService Nursing Research Program, Uniformed Services University of the Health Sciences, Landstuhl Regional Medical Center, the Department of Defense, or the U.S. Government.