BACKGROUND
Shoulder pain is among the most common musculoskeletal conditions in adults. Posterior shoulder tightness (PST), and associated glenohumeral internal rotation deficit (GIRD), is one cause of shoulder pain that has been consistently associated with shoulder pathology.1–6 PST may result from both muscular tightness and/or posterior capsular tightness,6–10 and manifests clinically as limited internal rotation (IR)11 and horizontal adduction range of motion (ROM).12 Posterior capsular tightness has been shown to increase antero-superior humeral head migration,13 potentially leading to impingement syndrome,12 while the posterior rotator cuff and posterior deltoid have also been described as potential sources of PST.6 Electromyographic activity of the posterior rotator cuff muscles has been shown to be elevated in individuals with shoulder pain14 and stiffness,15 and elevated levels of muscular activity can influence angular ROM.16 Clinically, other than non-validated end feel testing which clinically appears more elastic in the presence of muscle shortening and firmer in the presence of capsular shortening, one challenge is the inability to differentiate muscular and capsular tightness as a cause of PST17 and thus guide treatment. Overall, this distinction may not be possible, as the infraspinatus has been shown to be reflexively active in response to discharge of capsular afferents as part of the synergistic interplay of static and dynamic stabilizers,18,19 and a combined source of PST is likely in most individuals.6
Posterior glide mobilizations (PGM) have been demonstrated to improve ROM in patients with a variety of shoulder pathologies.11,20 PGM are an anterior-to-posterior directed force applied to the humeral head, designed to translate the humeral head posteriorly within the glenoid fossa. Improved ROM may be due to capsular stretch, as the ability of PGM to produce a tensile load on the posterior capsule has been well established.21–23 Prolonged bouts of joint mobilization may also produce neuromuscular changes, including decreased local resting electromyographic (EMG) activity.24 Previous authors have demonstrated improved humeral head translation accompanied by decreased EMG activity of the infraspinatus following sustained or oscillatory grade III PGM.15 Accordingly, improvements observed post-mobilization may be, at least partially, the result of decreased muscular activity.
Stretching techniques are also well supported in the literature to improve shoulder ROM,2,25 commonly assumed to target elongation of the local musculature. Decreased neuromuscular activity following stretching in individuals with pathology is also well documented.26 Static stretching appears to have an inhibitory effect on the involved muscles, although this effect has not been seen consistently in healthy individuals.27 Static stretching has also been shown to result in decreased force output and decreased EMG activity of the involved muscle group(s).27 Stretching the muscles of the rotator cuff may decrease their overall resistance to humeral head translation. However, the effects of stretching interventions on translatoric mobility within the shoulder and EMG activity of the rotator cuff have not been established.
Thoracic manipulation is commonly included in the treatment of individuals with shoulder pathology although systematic reviews offer conflicting conclusions regarding the effects in this population.28,29 Mintken et al. found baseline IR passive range of motion (PROM) limited to <53°, as is observed in individuals with PST, to be predictive as part of a test battery to determine likelihood of success for individuals with shoulder pain receiving cervicothoracic manual therapy including thoracic manipulation,30 and da Silva et al. observed increased shoulder flexion and abduction ROM in individuals with shoulder pain following a single T4-5 manipulation.31 Prior researchers have suggested that observed functional and GH ROM changes following thoracic manipulation are likely not due to mechanical changes, i.e. alterations of thoracic mobility or scapular kinematics.32 Rather, spinal manipulation is generally believed to cause a wide array of neurophysiologic effects, including either muscular excitation or inhibition.33,34 It is possible that the observed functional improvements observed following thoracic manipulation are due to neurophysiologic effects, potentially including some combination of decreased posterior rotator cuff activity and/or alterations in the afferent discharge from the glenohumeral capsule, and it is currently unclear if thoracic manipulation will result in decreased infraspinatus EMG activity, along with increased shoulder IR PROM and posterior joint mobility, in individuals with a loss of IR PROM.
In clinical practice, each of these techniques are widely utilized, and often in combination. However, the authors are not aware of any trials reporting a comparison of the immediate effects of the PGM and sleeper stretches in conjunction with thoracic manipulation on PROM, humeral head translation, and EMG activity in individuals who lack glenohumeral IR PROM. The authors hypothesized that the addition of thoracic manipulation would result in inhibition of the infraspinatus and be accompanied by greater gains in ROM and translation following PGM or stretching when compared to PGM or stretching alone. The purpose of this study was to assess the effects of adding thoracic manipulation to PGM and sleeper stretches on PROM, joint mobility, and infraspinatus EMG activity in shoulders with decreased IR PROM.
METHODS
This study utilized an assessor blinded, sequential, quasi-experimental repeated measures design that occurred in the human performance lab at the University of Hartford, West Hartford, CT, USA between March 31 and June 30, 2021. The study was approved by the University of Hartford Institutional Review Board, and prospectively registered at clinicaltrials.gov, NCT04777370.
Of the variables considered for this study, while IR PROM was considered the primary outcome of interest, EMG activity had the smallest reported effect size. Therefore, the sample size was determined using the differences in EMG activity observed in prior studies. Utilizing an α=0.05, 1-β=0.80, and a 5% difference in EMG activity between groups as observed by Muth35 and a standard deviation of ±5 as reported by Dunning and Rushton,36 a minimum of 16 subjects per group were required. To account for potential attrition and to ensure adequate sample size, 40 individuals (20 per combined intervention group) were enrolled.
A convenience sample of individuals who self-identified as having limited IR ROM was recruited via flyer, email, and word of mouth at the University of Hartford.
All participants were screened for inclusion/exclusion criteria via questionnaire and a brief screening exam. Volunteers were included if they were between the ages of 18-60 years old, and presented with a loss of GH IR PROM greater than 15° at 90° of shoulder abduction while seated with the scapula manually stabilized compared to the contralateral side.2
Consistent with prior studies examining the shoulder and thoracic spine,37,38 individuals were excluded if they reported any of the following conditions: current neck or upper back pain; prior shoulder, neck or upper back surgery; any previous injury to the neck or thoracic area; active inflammatory disease; osteoporosis; signs/symptoms of radiculopathy; upper motor neuron lesions; spinal cord pathology; local infection; active or history of cancer; long term corticosteroid use; systemically unwell; systemic hypermobility; connective tissue disease; pregnancy or recent pregnancy; blood clotting disorder; receiving workman’s compensation or involved in active litigation; or any other known contraindication to manual therapy.
Participants were randomized to either the mobilization or stretching intervention during the first session. A blinded third party prepared a set of sealed, opaque envelopes containing the intervention; participants selected an envelope and allocation was revealed to the examiner immediately prior to the intervention. During the second session, all participants received the thoracic manipulation intervention followed by the previously selected mobilization or stretching intervention.
All outcome measures were assessed by research associates blinded to group allocation.
Internal rotation PROM at 90° abduction: IR PROM of the involved shoulder was assessed in a seated position at 90 degrees abduction, with the scapula manually stabilized9 (MDC90: <5.5°).38 Measurements were performed using the inclinometer app for Android; smartphone based inclinometers have demonstrated excellent agreement with goniometry for ROM measurements of the shoulder (SEM overall: 3.6°, IR at 90° abd: 6.3°).39 Two trials were performed both pre-post intervention with the mean ROM used for analysis.
Horizontal adduction PROM: PST was assessed with the participant side-lying on the uninvolved side with the hips and knees flexed,1 as humeral horizontal adduction motion is suggested to be the most consistent indicator of posterior shoulder mobility deficits.40 The participants arm was placed in 90° abduction and neutral rotation and grasped by the researcher just distal to the humeral epicondyles.8 The scapula was stabilized by the researcher and the arm was passively brought into maximal horizontal adduction, defined as the point at which movement ceased or the researcher could no longer stabilize the scapula1 (SEM 3°, MDC90 8°).41 Two trials were performed both pre-post intervention; measurements were performed using the inclinometer app for Android with the mean ROM used for analysis.
Electromyography (EMG): Muscle activity of the infraspinatus was collected using a Delsys Trigno EMG system (Delsys, Boston, MA) via surface electrodes. After exposing the posterior shoulder girdle, the area of the infraspinatus was cleaned using a cloth alcohol prep pad, and the skin at the area of electrode placement was vigorously abraded for 5 seconds. A calibrated wireless electrode was then placed according to SENIAM guidelines.42 Once electrode placement was complete, each participant performed a standardizing reference contraction of the infraspinatus to ensure optimal EMG activity. The infraspinatus Maximal Voluntary Isometric Contraction (MVIC) was performed in standing, with the participant’s elbow against their side and flexed to 90°, and their distal forearm in neutral placed against a wall. The participant was asked to provide their maximal effort into external rotation against the wall for a period of five seconds. The peak activity during MVIC was selected for analysis using EMGworks (Delsys, Boston, MA) software.
Participants were then positioned supine on a standard plinth. To assess muscular activity during posterior humeral head translation, all participants received a 15 second sustained grade III PGM serving as the reference mobilization,43 with concurrent EMG and ultrasound data collection. To minimize motion artifact, the middle five seconds were utilized as the epoch of interest for EMG analysis. Reference mobilizations occurred at two time points: immediately pre- and immediately post-intervention.
Prior to analysis, all EMG data were RMS filtered using EMGworks software to create linear envelopes and normalized to the MVIC. Intraday EMG assessment of the infraspinatus using sub-maximal contraction has been shown to be reliable (ICC=0.98)44 and SEM values of 3.2-6.4% have been reported for surface EMG of the infraspinatus.45 Mean and peak values of infraspinatus activity during the reference mobilizations were calculated and compared between conditions during analysis.
Humeral Head Translation: A SonoSite MiniMaxx musculoskeletal ultrasound (US) unit (SonoSite, Bothell, WA) with a 5-11MHz linear transducer was utilized to measure the amount of humeral head translation occurring in the shoulder joint during the PGM techniques. US imaging has been shown to be a reliable measure of posterior translation of the humeral head during mobilization.46 The transducer was oriented horizontally over the anterior shoulder visualizing the coracoid process, the lesser tuberosity, and the biceps tendon in the display. (Figure 1) With the arm held in the GH resting position, a resting image was taken. The examiner then performed a PGM of the shoulder (static x 15 seconds) and a second image was obtained. For each image, a measurement of the distance between the most anterior aspect of the coracoid and the most anterior aspect of the lesser tuberosity was obtained. The amount of posterior humeral head translation was determined by subtracting the distance at rest from the distance during mobilization. Measures of humeral head position assessing anterior landmarks have been shown to have high levels of intra-tester reliability (ICC 0.93), SEM 0.5-1.0mm, SDD 1.6-2.7mm.47
Interventions
Mobilization: All interventions were performed by an experienced therapist with fellowship training in manual therapy. The participant was positioned supine on a plinth, with their scapula stabilized against a firm wedge on the table, and the shoulder joint in the resting position (approx. 55° abduction, 30° horizontal adduction, and slight external rotation). With the extremity held in the same position, the researcher applied five 30-second bouts of sustained grade III PGM.15
Stretching: All participants randomized to the stretching group performed five 30-second holds of the sleeper stretch. This was performed by lying on the side to be stretched, elevating the upper arm to 90° on the support surface with the elbow bent 90°, then passively internally rotating the shoulder with force provided by with the opposite arm.2 Participants were instructed to push to the point of moderate-to-strong stretch within their tolerance and no more than mild discomfort (<4/10).
Thoracic manipulation: All individuals received a single supine grade V thrust manipulation as described by Cleland,48,49 localized to the T3-4 segment. The supine technique was selected as it has been shown to elicit a greater change in pain when compared to seated techniques,50 and is more readily applied to the upper thoracic region than prone techniques. If a cavitation (“pop”) was not heard or felt by either the subject or examiner, a second thrust was performed.
Participant flow
Session #1: Baseline data collection → randomized intervention → post-intervention data collection → 7-day washout period51,52
Session #2: Baseline data collection → Thoracic manipulation → post-manipulation data collection → randomized intervention → post-intervention data collection
Based on the findings of Wang and Meadows, the effects of spinal manipulation on the shoulder last greater than 10 but less than 20 minutes post intervention.53 Therefore, all additional interventions and assessment took place within a 10-minute timeframe following the initial thoracic manipulation. The results of the final measurements taken during session two were compared to the results of session one to assess the additive effect of the thoracic manipulation.
Data analysis
All data were analyzed quantitatively in aggregate form using SPSS (IBM SPSS 25, Armonk, NY) and descriptive statistics were calculated using Microsoft Excel. The level of significance was established a priori with the α value set to .05 and the β set at 0.2.
Dependent variables included changes in IR and horizontal adduction ROM, change in humeral head translation, and change in EMG activity during reference PGM. Analysis followed intention-to-treat principles, and any missing data were imputed using baseline observations carried forward as a conservative estimate of effect.54 All data were assessed for normality using the Shapiro-Wilk test, visual inspection of the Q-Q plot and the Levene statistic for homogeneity of variance. Analyses were completed with and without outliers, defined as data points beyond the 95th percentile. No outliers materially affected the results, and therefore remained as the observed values best represent the sample characteristics.
Between group differences were assessed with repeated measures analysis of variance (ANOVA), pairwise testing, and post-hoc testing as appropriate. Simple between group comparisons were assessed with independent t-tests, and within group comparisons were assessed with paired t-tests. As participants served as their own controls, the cumulative effects of the combined thoracic manipulation and mobilization/stretching intervention were compared to the single intervention session results. Effect sizes were calculated using the Cohen’s d statistic; effect sizes d=0.2 were considered small, d=0.5 medium, and d=0.8 large. Between session analyses of EMG data were not performed, as between session reliability of surface EMG is modest at best.55
RESULTS
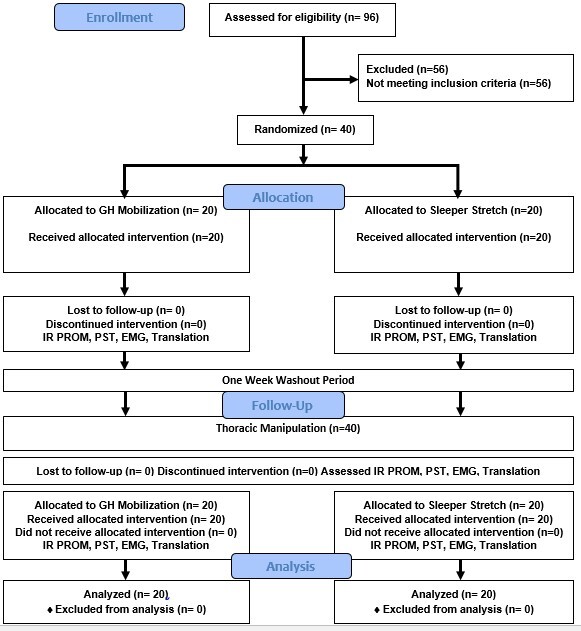
Participant flow is detailed in Figure 2; demographics and baseline characteristics are shown in Table 1. There were no adverse events reported at any time during this study.
Session 1
Following single interventions (PGM, sleeper stretching) no significant differences were observed between groups for IR ROM, horizontal adduction ROM, humeral head translation, or EMG activity. However, both interventions resulted in statistically significant within group changes (p<0.0001) with large effect sizes for IR (PGM 8.8±5.5°, 95% CI [6.4, 11.2], d=1.6; Sleeper 10.0±4.9°, 95% CI [7.9, 12.2], d=2.02) and horizontal adduction ROM (PGM 5.2±4.5°, 95% CI [3.2,7.2] d=1.15; Sleeper 3.1±2.1°, 95% CI [2.2,4.0], d=1.48) which also exceeded the SEM of the measures and IR ROM exceeding the MDC90 for both groups.
Changes in humeral head translation were observed within each group that were not intuitive. The change in measured excursion [(end position2 – starting position2)-(end position1 – starting position1)] resulted in significant within group differences for the sleeper stretch (1.72±2.93mm, 95% CI 0.4, 3.0], p=0.017, d=0.59) but not the PGM (1.23±3.56mm, 95% CI [-0.3, 2.8], p=0.138, d=0.35). However, the PGM resulted in a significant change (2.01±2.94mm, 95% CI [0.72, 3.30], p=.006, d=0.68) in posterior positioning of the humeral head (starting position2-starting position1) that was not observed for the sleeper stretch group (0.54±1.86mm, 95% CI [-0.28, 1.35], p=0.209, d=0.29) and exceeded the SEM and SDD of the measure. When both change in starting position and excursion were considered, there were significant within group changes for total posterior humeral head translation following both interventions (PGM 3.23±2.77mm, 95% CI [2.02, 4.44], p<0.0001, d=1.17; sleeper 2.68±2.77mm, 95% CI [1.47, 3.89] p=0.0003, d=0.97). EMG activity decreased following both interventions, but changes were not significantly different within (PGM -0.50±1.30%, 95% CI [-1.07, 0.07], p=0.102; sleeper -0.17±0.57%, 95% CI [-0.42, 0.08], p=0.209) or between groups (0.33%, p=0.305). (Table 2, Figure 3)
Session 2
Thoracic manipulation vs Single Interventions
There were small carryover effects for baseline IR ROM (1.9°) and horizontal adduction ROM (0.5°) between Session 1 and Session 2 that were smaller than possible measurement error and did not reach statistically significant differences. There was a significant difference in baseline GH translation between Session 1 and Session 2 (2.0mm); this difference was significant for both the PGM (2.1±4.0mm, 95% CI[0.2, 4.0], p=0.03) and sleeper stretch (2.0±3.4, 95% CI[0.4, 3.6], p=0.019) groups and was within the proposed range of SDD for the measurement.
IR PROM
Thoracic manipulation resulted in a small increase in IR ROM (0.4°±4.5, 95% CI [-1.0, 1.8], p=0.539) that was not statistically significant. There were significant between group differences following single interventions (mobilization or sleeper [session 1] and thoracic manipulation [session 2]), F1,38=60.55, p<0.001. Post-hoc testing revealed significant differences between PGM and thoracic manipulation, (mean difference -8.4°, 95% CI [-11.5,-5.3], p<0.001, d=1.67) and between sleeper stretching and thoracic manipulation, (mean difference -9.5°, 95% CI [-12.6, -6.4], p<0.001, d=2.04) with thoracic manipulation resulting in much smaller changes than the local shoulder interventions. (Table 3)
Horizontal Adduction PROM
Thoracic manipulation resulted in small changes in horizontal adduction ROM (0.6°±3.4, 95% CI [-0.5, 1.7], p=0.285) that were not statistically significant. There were significant between group differences following single interventions (mobilization or sleeper [Session 1] vs thoracic manipulation [Session 2]), F1,38=21.69, p<0.0001. Post-hoc testing revealed significant differences between PGM and thoracic manipulation, (mean difference -4.6°, 95% CI [-6.9, -2.3], p<0.0001, d=1.15) and between sleeper stretches and thoracic manipulation, (mean difference -2.5°, 95% CI [-4.7, -0.2], p=0.031, d=0.88). (Table 3)
EMG
Thoracic manipulation resulted in small changes in mean EMG activity of the infraspinatus that were not statistically significant (mean difference -0.07±0.30, 95% CI [-0.03, 0.16], p=0.177, d=0.218]. Peak EMG demonstrated statistically significant changes that did not exceed SEM (mean difference 0.41±0.94% MVIC, 95% CI [0.11, 0.71], p=0.008, d=0.44). (Table 3)
Humeral Head Translation
Thoracic manipulation resulted in small changes in humeral head translation (0.3±3.0mm, 95% CI [-0.63, 1.23], p=.517) that were not statistically significant. For change in humeral head translation, there were significant between group differences for single interventions (mobilization or sleeper [Session 1] vs thoracic manipulation [Session 2]), F1,39=9.60, p=0.004. Post-hoc testing revealed significant differences between PGM and thoracic manipulation (mean difference -1.7±3.1mm, 95% CI [-3.1, -0.2], p=0.028, d=0.58) while between group differences for sleeper stretches and thoracic manipulation did not reach statistically significant differences (mean difference -1.8±4.0mm, 95% CI [-3.7, 0.1], p=0.057, d=0.66). (Table 3)
Combined Interventions
IR PROM
There were significant differences in IR ROM between single and combined interventions, F1,38=42.17, p<0.001. When combined with thoracic manipulation, both PGM and sleeper stretches resulted in significantly smaller within session changes for IR ROM compared to single interventions: PGM (mean difference 4.4°±7.5, 95% CI [0.9, 7.9] p=0.017, d=0.77); sleeper stretch (mean difference 6.4°±6.9, 95% CI [3.2, 9.6] p=0.0005, d=1.32). (Table 3, Figure 5)
Horizontal Adduction PROM
Across all participants, there were significant differences in horizontal adduction ROM between single and combined interventions, F1,38=12.53, p=0.001. When combined with thoracic manipulation, both PGM and sleeper stretches resulted in smaller within session changes compared to single interventions that were not statistically significant: PGM (mean difference 2.6°±5.7, 95% CI [-0.04, 5.2] p=0.054, d=0.63); sleeper stretch (mean difference 1.5°, 95% CI [-0.9, 3.9] p=0.199, d=0.44). (Table 3, Figure 5)
EMG
When combined with thoracic manipulation, subsequent PGMs resulted in no further decrease in peak (mean difference -.02%±0.44% MVIC, 95% CI [-0.22, 0.19], p=.876, d=.04) and mean (mean difference .001±0.17% MVIC, 95% CI [-0.08, .08], p=0.979, d=.01) EMG activity. When combined with thoracic manipulation, subsequent sleeper stretches resulted in further reductions in both peak (mean difference 0.21±0.68% MVIC, 95% CI [-0.11, 0.53], p=0.188, d=0.31) and mean (mean difference 0.16±0.30% MVIC, 95% CI [0.02, 0.30], p=.027, d=0.53) EMG activity. (Table 3, Figure 4)
Humeral Head Translation
When combined with thoracic manipulation, changes in humeral head translation were smaller compared to mobilization or stretching alone: PGM (mean difference 0.26mm, 95% CI [-1.83, 2.35] p=0.775, d=0.08); sleeper stretch (mean difference 0.66mm, 95% CI [-1.07, 2.39] p=0.450, d=0.24), differences that were not statistically significantly different. (Table 3, Figure 5)
DISCUSSION
Overall, there were no significant differences in effect between five bouts of PGM and five bouts of sleeper stretching for IR ROM, horizontal adduction ROM, or humeral head translation. Change exceeded measurement error for each of these outcomes, suggesting that both interventions were helpful in achieving the desired outcomes. While both horizontal adduction and IR assess the motion of both the posterior muscles and posterior capsule,8 the greater observed improvement of horizontal adduction following PGM may be due to a more direct mechanical influence of PGM on the posterior capsule where mechanically the mobilization more closely approximates the test. Application of PGM at the resting position rather than in progressive end range motion may have limited the overall ROM gains observed.
Conversely, the sleeper stretch resulted in slightly greater IR gains than the PGM. The sleeper stretch is a more direct analog to the IR PROM test, with the greatest strain on the inferior fibers of the infraspinatus occurring in this position.56 Both PGM and sleeper stretches resulted in decreased EMG activity of the infraspinatus. This finding is in agreement with the conclusions of the recent systematic review by Pfleuger et al., who reported finding moderate quality evidence that “(peripheral) joint mobilization immediately decreases the activation of superficial muscles during low load conditions in symptomatic individuals”57 while also in concordance with studies regarding muscular inhibition following static stretching.27 Accordingly, clinicians should expect to see improvements in IR PROM following these interventions.
Contrary to the initial hypothesis, the addition of thoracic manipulation prior to mobilization or stretching resulted in significantly smaller gains in IR PROM which exceeded potential measurement error compared to mobilization or stretching alone. Changes in resting position were greater following combined interventions while infraspinatus EMG activity decreased following the combined interventions. Overall interpretation of the impact of combined interventions on GH translation is limited, as there were significant differences at baseline between session #1 and session #2. However, the overall difference in translation between sessions was smaller than SDD for the measure and accompanied by a difference in IR PROM of less than 2°. Accordingly, the observed 2mm difference may not be of clinical significance. It appears unlikely that the observed differences in ROM between single and combined interventions were due to differences in humeral head translation or infraspinatus EMG activity.
From the current study, it is not entirely clear which muscles or mechanisms were responsible for limiting the IR PROM gains post thoracic manipulation. Previous work has demonstrated an increase in distal muscular activity following manipulation,36,53,58 and it is possible that thoracic manipulation had an excitatory effect on the middle deltoid58,59 or other shoulder musculature. For example, Hawkes et al. observed an increase in teres minor and latissimus dorsi activation in concert with deltoid contraction in individuals with rotator cuff pathology, proposed to be a means to decrease humeral head translation.60 Given the apparent lack of linear relationship between infraspinatus activity, GH translation, and IR PROM observed in this study, the assessment of only a single RC muscle is a clear limitation of this research, and further research is required to determine which muscles are responsible for the decrease in PROM observed following thoracic manipulation.
From a neurophysiologic perspective, when considering that the addition of PGM following thoracic manipulation resulted in no further changes in EMG activity of the infraspinatus, it appears that PGM and thoracic manipulation may function through similar pathways/mechanisms. Following mobilization/manipulation, centrally mediated reflex arcs or changes in the sensitivity of the α-motoneurons have been described.61 Proprioceptive input comes from stretch sensitive mechanoreceptors in the joint capsule, muscle spindles, and from the Golgi tendon organs of local musculature, which is then mediated by the dorsal root ganglion.62 Fisher et al. suggested that high velocity manipulation appears to generate a supraspinal response, while changes following low velocity mobilization were likely the result of reduced spinal excitability.63 Contrary to their conclusions, in the current study, it appears that thoracic manipulation (high velocity) and PGM (low velocity) may have influenced a similar pathway as PGM generated no further inhibitory effect at the infraspinatus following thoracic manipulation. This discrepancy may be due to the presence of few mechanoreceptors in the shoulder capsule/ligaments,62 and responses to manual therapy may be region/tissue dependent. It is also possible that altered stretch tolerance is the result of changes to the input to nociceptive nerve endings in the joint and muscle.64 If the reduction in reflexive contraction during mobilization is due to altered nociceptive response, the current findings align with those of Coronado et al., who found a non-specific pain reduction effect at the shoulder that did not differ between cervical and shoulder thrust manipulation.65 However, the relation between the delivered manual therapy dose and subsequent treatment outcome remains unknown,66 and it may be plausible that there is not further neurophysiologic effect to be gained from further glenohumeral mobilizations following thoracic HVLA manipulation.
Conversely, stretching, and thoracic manipulation may influence different mechanisms/pathways as sleeper stretches, but not PGM, resulted in further reductions of EMG activity following thoracic manipulation. Previous research has suggested that stretching results in decreased EMG activity, likely via altered reflex sensitivity67 involving the fusiform/muscle spindle system.68 The discharge of muscle spindle endings is affected by local muscular stretch,69 while the γ-motoneuron controls the sensitivity of muscle spindle afferents as length detectors.70 Changes in γ-motoneuron activity may result in changes in 1a afferent activity and decreased α-motoneuron output.71 These apparent differences in mechanism of action between manipulation/mobilization and stretching support the concept of combined interventions with the intention of improving IR ROM, and may help explain the additive benefits of PGM and sleeper stretching observed previously.11
There is conflicting evidence regarding thoracic manipulation for individuals with shoulder dysfunction. Improvement following thoracic manual therapy has been observed in a case series of individuals with shoulder pain.72 Prior studies have demonstrated a short-term increase in scapular muscle strength, including the middle35 and lower trapezius.73 However, the observed improvements are not accompanied by changes in scapular mechanics,74 and the inclusion of thoracic manipulation vs sham manipulation may not influence outcomes for individuals with shoulder impingement.75 A recent systematic review concluded that that manipulation of the thoracic spine has questionable effectiveness when compared to other interventions for improving pain and function for individuals with upper quarter musculoskeletal dysfunctions.76 Considering the results of the current study in the context of this previous research, the authors suggest the effect of thoracic manipulation is not one size fits all, but rather should be tailored to fit the clinical goals. If the clinical goal is to decrease pain28 or improve middle/lower trapezius recruitment, then thoracic manipulation may be indicated.73,77 However, if the primary impairment is limited IR PROM with the clinical goal to improve ROM, thoracic manipulation may at best have little benefit, or at worst be counter-productive. In this instance, it appears that either PGM or sleeper stretches would yield greater benefits.
There are several limitations to consider in the interpretation of these results. First, the sample was comprised of individuals with non-clinical shoulder stiffness, and most reported very low levels of pain. Since individuals with higher levels of pain have been shown to have higher levels of posterior rotator cuff EMG activity during PGM,14 a pain dominant sample may present with different results. Inclusion was based on the presence of a significant IR PROM loss. The screening and inclusion/exclusion did not account for the possibility of osseous limitations. It is possible that individuals within the study presented with IR PROM loss due to humeral torsion or other osseous limitations which would limit the individual’s ability to demonstrate change post intervention. The EMG measures only assessed the infraspinatus, and only during PGM. Based on the results, it appears clear that assessment of a greater range of shoulder muscles is required to elucidate the source of decreased ROM after the inclusion of thoracic manipulation, and that these muscles should be assessed during IR ROM measurements as well. Thoracic manipulation was only applied at one prescriptive spinal level. While prescriptive application improves internal validity of the study in answering the question of manipulative force applied to the upper thoracic region, this is not how the techniques are generally applied clinically. It is not known if individuals would respond differently if the manipulation were applied at pragmatically identified symptomatic stiff and/or painful levels of the thoracic spine. It remains unknown whether the observed 2mm change in baseline translation between sessions was clinically meaningful, although the changes in translation were accompanied by very small changes in angular motion, and therefore appear unlikely to be meaningful. Further, while performed for a duration that is substantially less than general clinical application, the possibility that the initial PG used to determine baseline translation and EMG activity resulted in a treatment effect cannot be eliminated.
Conclusion
As expected, both GH posterior mobilizations and sleeper stretches improved both IR and horizontal adduction PROM. The addition of thoracic manipulation prior to local shoulder intervention resulted in progressive reductions of infraspinatus EMG activity but also a reduction in ROM gains for both IR and horizontal adduction. These findings suggest that if the therapeutic intent is to improve IR ROM in individuals with non-painful, stiff shoulders, the addition of thoracic manipulation may be counterproductive.
Funding
This work was supported in part by a University of Hartford Greenberg Junior Faculty Grant. This support does not necessarily imply endorsement by the University of Hartford of project conclusions.

_pre-post_single_intervention.png)
_pre-post_thoracic_manipulation___combined_interventions.png)


_pre-post_single_intervention.png)
_pre-post_thoracic_manipulation___combined_interventions.png)
